Development of Pandemic Vaccines: ERVEBO Case Study
- PMID: 33668698
- PMCID: PMC7996233
- DOI: 10.3390/vaccines9030190
Development of Pandemic Vaccines: ERVEBO Case Study
Abstract
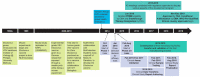
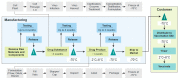
Preventative vaccines are considered one of the most cost-effective and efficient means to contain outbreaks and prevent pandemics. However, the requirements to gain licensure and manufacture a vaccine for human use are complex, costly, and time-consuming. The 2013-2016 Ebola virus disease (EVD) outbreak was the largest EVD outbreak to date and the third Public Health Emergency of International Concern in history, so to prevent a pandemic, numerous partners from the public and private sectors combined efforts and resources to develop an investigational Zaire ebolavirus (EBOV) vaccine candidate (rVSVΔG-ZEBOV-GP) as quickly as possible. The rVSVΔG-ZEBOV-GP vaccine was approved as ERVEBOTM by the European Medicines Authority (EMA) and the United States Food and Drug Administration (FDA) in December 2019 after five years of development. This review describes the development program of this EBOV vaccine, summarizes what is known about safety, immunogenicity, and efficacy, describes ongoing work in the program, and highlights learnings applicable to the development of pandemic vaccines.
Keywords: Ebolavirus vaccine; rVSVΔG-ZEBOV-GP; regulatory strategy; vaccine development; vaccine manufacturing.
Conflict of interest statement
All authors are employees of MSD and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Figures

References
-
- World Health Organization . Ebola Strategy: Ebola and Marburg Virus Disease Epidemics: Preparedness, Alert, Control, and Evaluation. WHO; Geneva, Switzerland: 2014.
-
- Gouglas D., Le T.T., Henderson K., Kaloudis A., Danielsen T., Hammersland N.C., Robinson J.M., Heaton P.M., Røttingen J.-A. Estimating the cost of vaccine development against epidemic infectious diseases: A cost minimisation study. Lancet Glob. Health. 2018;6:e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. - DOI - PMC - PubMed
-
- Hurford P., Davis M.A. How much does it cost to research and develop a vaccine? [(accessed on 18 February 2021)];Eff. Altruism Forum. 2018 Available online: https://forum.effectivealtruism.org/posts/BjBmcfwg2awqPJLin/how-much-doe....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

