Circadian Rhythms of the Hypothalamus: From Function to Physiology
- PMID: 33668705
- PMCID: PMC7931002
- DOI: 10.3390/clockssleep3010012
Circadian Rhythms of the Hypothalamus: From Function to Physiology
Abstract
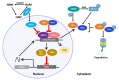
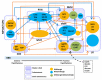
The nearly ubiquitous expression of endogenous 24 h oscillations known as circadian rhythms regulate the timing of physiological functions in the body. These intrinsic rhythms are sensitive to external cues, known as zeitgebers, which entrain the internal biological processes to the daily environmental changes in light, temperature, and food availability. Light directly entrains the master clock, the suprachiasmatic nucleus (SCN) which lies in the hypothalamus of the brain and is responsible for synchronizing internal rhythms. However, recent evidence underscores the importance of other hypothalamic nuclei in regulating several essential rhythmic biological functions. These extra-SCN hypothalamic nuclei also express circadian rhythms, suggesting distinct regions that oscillate either semi-autonomously or independent of SCN innervation. Concurrently, the extra-SCN hypothalamic nuclei are also sensitized to fluctuations in nutrient and hormonal signals. Thus, food intake acts as another powerful entrainer for the hypothalamic oscillators' mediation of energy homeostasis. Ablation studies and genetic mouse models with perturbed extra-SCN hypothalamic nuclei function reveal their critical downstream involvement in an array of functions including metabolism, thermogenesis, food consumption, thirst, mood and sleep. Large epidemiological studies of individuals whose internal circadian cycle is chronically disrupted reveal that disruption of our internal clock is associated with an increased risk of obesity and several neurological diseases and disorders. In this review, we discuss the profound role of the extra-SCN hypothalamic nuclei in rhythmically regulating and coordinating body wide functions.
Keywords: circadian rhythm; clock genes; extra-SCN hypothalamic nuclei; food-entrainable oscillator; hypothalamus; metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

