Imaging Techniques to Study Plant Virus Replication and Vertical Transmission
- PMID: 33668729
- PMCID: PMC7996213
- DOI: 10.3390/v13030358
Imaging Techniques to Study Plant Virus Replication and Vertical Transmission
Abstract
Plant viruses are obligate parasites that need to usurp plant cell metabolism in order to infect their hosts. Imaging techniques have been used for quite a long time to study plant virus-host interactions, making it possible to have major advances in the knowledge of plant virus infection cycles. The imaging techniques used to study plant-virus interactions have included light microscopy, confocal laser scanning microscopy, and scanning and transmission electron microscopies. Here, we review the use of these techniques in plant virology, illustrating recent advances in the area with examples from plant virus replication and virus plant-to-plant vertical transmission processes.
Keywords: correlative microscopy; electron microscopy; imaging; immunocytochemistry; in situ hybridization; light microscopy; plant virus–host interactions; viral life cycle; viral replication; viral transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

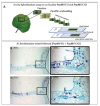
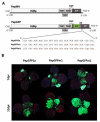
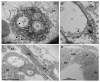


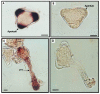
References
-
- Gosalvez-Bernal B., Garcia-Castillo S., Pallás V., Sanchez-Pina M. Distribution of carnation viruses in the shoot tip: Exclusion from the shoot apical meristem. Physiol. Mol. Plant Pathol. 2006;69:43–51. doi: 10.1016/j.pmpp.2006.12.004. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

