The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection
- PMID: 33668777
- PMCID: PMC7996247
- DOI: 10.3390/v13030359
The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection
Abstract
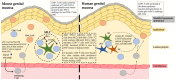
Tissue-resident memory T cells (TRM) were first described in 2009. While initially the major focus was on CD8+ TRM, there has recently been increased interest in defining the phenotype and the role of CD4+ TRM in diseases. Circulating CD4+ T cells seed CD4+ TRM, but there also appears to be an equilibrium between CD4+ TRM and blood CD4+ T cells. CD4+ TRM are more mobile than CD8+ TRM, usually localized deeper within the dermis/lamina propria and yet may exhibit synergy with CD8+ TRM in disease control. This has been demonstrated in herpes simplex infections in mice. In human recurrent herpes infections, both CD4+ and CD8+ TRM persisting between lesions may control asymptomatic shedding through interferon-gamma secretion, although this has been more clearly shown for CD8+ T cells. The exact role of the CD4+/CD8+ TRM axis in the trigeminal ganglia and/or cornea in controlling recurrent herpetic keratitis is unknown. In HIV, CD4+ TRM have now been shown to be a major target for productive and latent infection in the cervix. In HSV and HIV co-infections, CD4+ TRM persisting in the dermis support HIV replication. Further understanding of the role of CD4+ TRM and their induction by vaccines may help control sexual transmission by both viruses.
Keywords: HIV-1; HSV-1/2; immunity; infection; keratitis; tissue resident CD4+, CD8+, vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kumar B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J., Senda T., Sun X., Ho S.H., Lerner H., et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. - DOI - PMC - PubMed
-
- Wong M.T., Ong D.E., Lim F.S., Teng K.W., McGovern N., Narayanan S., Ho W.Q., Cerny D., Tan H.K., Anicete R., et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity. 2016;45:442–456. doi: 10.1016/j.immuni.2016.07.007. - DOI - PubMed
-
- Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J.J., Bickham K.L., Lerner H., Goldstein M., Sykes M., et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. - DOI - PMC - PubMed
-
- Yang Q., Zhang M., Chen Q., Chen W., Wei C., Qiao K., Ye T., Deng G., Li J., Zhu J., et al. Cutting Edge: Characterization of Human Tissue-Resident Memory T Cells at Different Infection Sites in Patients with Tuberculosis. J. Immunol. 2020;204:2331–2336. doi: 10.4049/jimmunol.1901326. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

