Interactions between Growth of Muscle and Stature: Mechanisms Involved and Their Nutritional Sensitivity to Dietary Protein: The Protein-Stat Revisited
- PMID: 33668846
- PMCID: PMC7996181
- DOI: 10.3390/nu13030729
Interactions between Growth of Muscle and Stature: Mechanisms Involved and Their Nutritional Sensitivity to Dietary Protein: The Protein-Stat Revisited
Abstract
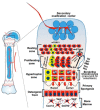
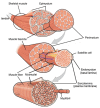
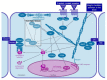
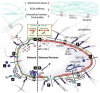
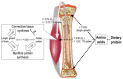
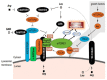
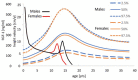
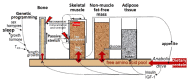
Childhood growth and its sensitivity to dietary protein is reviewed within a Protein-Stat model of growth regulation. The coordination of growth of muscle and stature is a combination of genetic programming, and of two-way mechanical interactions involving the mechanotransduction of muscle growth through stretching by bone length growth, the core Protein-Stat feature, and the strengthening of bone through muscle contraction via the mechanostat. Thus, growth in bone length is the initiating event and this is always observed. Endocrine and cellular mechanisms of growth in stature are reviewed in terms of the growth hormone-insulin like growth factor-1 (GH-IGF-1) and thyroid axes and the sex hormones, which together mediate endochondral ossification in the growth plate and bone lengthening. Cellular mechanisms of muscle growth during development are then reviewed identifying (a) the difficulties posed by the need to maintain its ultrastructure during myofibre hypertrophy within the extracellular matrix and the concept of muscle as concentric "bags" allowing growth to be conceived as bag enlargement and filling, (b) the cellular and molecular mechanisms involved in the mechanotransduction of satellite and mesenchymal stromal cells, to enable both connective tissue remodelling and provision of new myonuclei to aid myofibre hypertrophy and (c) the implications of myofibre hypertrophy for protein turnover within the myonuclear domain. Experimental data from rodent and avian animal models illustrate likely changes in DNA domain size and protein turnover during developmental and stretch-induced muscle growth and between different muscle fibre types. Growth of muscle in male rats during adulthood suggests that "bag enlargement" is achieved mainly through the action of mesenchymal stromal cells. Current understanding of the nutritional regulation of protein deposition in muscle, deriving from experimental studies in animals and human adults, is reviewed, identifying regulation by amino acids, insulin and myofibre volume changes acting to increase both ribosomal capacity and efficiency of muscle protein synthesis via the mechanistic target of rapamycin complex 1 (mTORC1) and the phenomenon of a "bag-full" inhibitory signal has been identified in human skeletal muscle. The final section deals with the nutritional sensitivity of growth of muscle and stature to dietary protein in children. Growth in length/height as a function of dietary protein intake is described in the context of the breastfed child as the normative growth model, and the "Early Protein Hypothesis" linking high protein intakes in infancy to later adiposity. The extensive paediatric studies on serum IGF-1 and child growth are reviewed but their clinical relevance is of limited value for understanding growth regulation; a role in energy metabolism and homeostasis, acting with insulin to mediate adiposity, is probably more important. Information on the influence of dietary protein on muscle mass per se as opposed to lean body mass is limited but suggests that increased protein intake in children is unable to promote muscle growth in excess of that linked to genotypic growth in length/height. One possible exception is milk protein intake, which cohort and cross-cultural studies suggest can increase height and associated muscle growth, although such effects have yet to be demonstrated by randomised controlled trials.
Keywords: IGF-1; amino acids; bone; dietary-protein; growth; insulin; mechanotransduction; muscle; protein-synthesis; satellite-cells.
Conflict of interest statement
The author declares no conflict of interest. The author has received consulting, travel, and/or speaker fees and research support (last 5 years) from Abbott, Nestlé Health Science, Public Health England and the UK Ministry of Defence.
Figures








Similar articles
-
Post-natal muscle growth and protein turnover: a narrative review of current understanding.Nutr Res Rev. 2024 Jun;37(1):141-168. doi: 10.1017/S0954422423000124. Epub 2023 Jul 3. Nutr Res Rev. 2024. PMID: 37395180 Review.
-
Dynamics of muscle fibre growth during postnatal mouse development.BMC Dev Biol. 2010 Feb 22;10:21. doi: 10.1186/1471-213X-10-21. BMC Dev Biol. 2010. PMID: 20175910 Free PMC article.
-
Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children.Nutr Res Rev. 2017 Jun;30(1):50-72. doi: 10.1017/S0954422416000238. Epub 2017 Jan 23. Nutr Res Rev. 2017. PMID: 28112064 Review.
-
Associations of protein intake in early childhood with body composition, height, and insulin-like growth factor I in mid-childhood and early adolescence.Am J Clin Nutr. 2019 Apr 1;109(4):1154-1163. doi: 10.1093/ajcn/nqy354. Am J Clin Nutr. 2019. PMID: 30869114 Free PMC article. Clinical Trial.
-
Animal protein intakes during early life and adolescence differ in their relation to the growth hormone-insulin-like-growth-factor axis in young adulthood.J Nutr. 2013 Jul;143(7):1147-54. doi: 10.3945/jn.113.175877. Epub 2013 May 22. J Nutr. 2013. PMID: 23700336
Cited by
-
Targeting Sarcopenia as an Objective Clinical Outcome in the Care of Children with Spinal Cord-Related Paralysis: A Clinician's View.Children (Basel). 2023 May 5;10(5):837. doi: 10.3390/children10050837. Children (Basel). 2023. PMID: 37238385 Free PMC article. Review.
-
Protein Appetite at the Interface between Nutrient Sensing and Physiological Homeostasis.Nutrients. 2021 Nov 16;13(11):4103. doi: 10.3390/nu13114103. Nutrients. 2021. PMID: 34836357 Free PMC article. Review.
-
Nutrient intakes from complementary foods are associated with cardiometabolic biomarkers among undernourished Peruvian children.J Nutr Sci. 2023 Jul 19;12:e80. doi: 10.1017/jns.2023.66. eCollection 2023. J Nutr Sci. 2023. PMID: 37528831 Free PMC article.
-
Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss.Nutrients. 2021 Jul 20;13(7):2473. doi: 10.3390/nu13072473. Nutrients. 2021. PMID: 34371981 Free PMC article. Review.
-
Post-prandial tracer studies of protein and amino acid utilisation: what can they tell us about human amino acid and protein requirements?Br J Nutr. 2024 Jun 28;131(12):2005-2030. doi: 10.1017/S0007114524000734. Epub 2024 Apr 12. Br J Nutr. 2024. PMID: 38606599 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

