Embryonic Environmental Niche Reprograms Somatic Cells to Express Pluripotency Markers and Participate in Adult Chimaeras
- PMID: 33668852
- PMCID: PMC7996319
- DOI: 10.3390/cells10030490
Embryonic Environmental Niche Reprograms Somatic Cells to Express Pluripotency Markers and Participate in Adult Chimaeras
Abstract
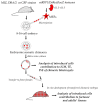
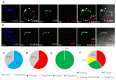
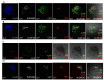
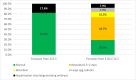
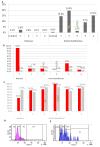
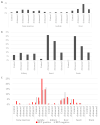
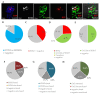
The phenomenon of the reprogramming of terminally differentiated cells can be achieved by various means, like somatic cell nuclear transfer, cell fusion with a pluripotent cell, or the introduction of pluripotency genes. Here, we present the evidence that somatic cells can attain the expression of pluripotency markers after their introduction into early embryos. Mouse embryonic fibroblasts introduced between blastomeres of cleaving embryos, within two days of in vitro culture, express transcription factors specific to blastocyst lineages, including pluripotency factors. Analysis of donor tissue marker DNA has revealed that the progeny of introduced cells are found in somatic tissues of foetuses and adult chimaeras, providing evidence for cell reprogramming. Analysis of ploidy has shown that in the chimaeras, the progeny of introduced cells are either diploid or tetraploid, the latter indicating cell fusion. The presence of donor DNA in diploid cells from chimaeric embryos proved that the non-fused progeny of introduced fibroblasts persisted in chimaeras, which is evidence of reprogramming by embryonic niche. When adult somatic (cumulus) cells were introduced into early cleavage embryos, the extent of integration was limited and only cell fusion-mediated reprogramming was observed. These results show that both cell fusion and cell interactions with the embryonic niche reprogrammed somatic cells towards pluripotency.
Keywords: chimaera; embryonic niche; plasticity; reprogramming.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Comment in
-
Introduction of Mouse Embryonic Fibroblasts into Early Embryos Causes Reprogramming and (Con)fusion.Cells. 2021 Mar 31;10(4):772. doi: 10.3390/cells10040772. Cells. 2021. PMID: 33807431 Free PMC article.
-
Introduction of Mouse Embryonic Fibroblasts into Early Embryos: From Confusion to Constructive Discussion. Comment on Savatier, P. Introduction of Mouse Embryonic Fibroblasts into Early Embryos Causes Reprogramming and (Con)fusion. Cells 2021, 10, 772.Cells. 2021 Jun 18;10(6):1534. doi: 10.3390/cells10061534. Cells. 2021. PMID: 34206996 Free PMC article.
References
-
- Waddington C.H. The Strategy of the Genes. George Allen and Unwin; London, UK: 1957.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

