Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis
- PMID: 33668983
- PMCID: PMC7956327
- DOI: 10.3390/ijms22052284
Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis
Abstract
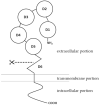
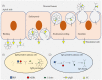
Transcytosis of polymeric IgA and IgM from the basolateral surface to the apical side of the epithelium and subsequent secretion into mucosal fluids are mediated by the polymeric immunoglobulin receptor (pIgR). Secreted IgA and IgM have vital roles in mucosal immunity in response to pathogenic infections. Binding and recognition of polymeric IgA and IgM by pIgR require the joining chain (J chain), a small protein essential in the formation and stabilization of polymeric Ig structures. Recent studies have identified marginal zone B and B1 cell-specific protein (MZB1) as a novel regulator of polymeric IgA and IgM formation. MZB1 might facilitate IgA and IgM transcytosis by promoting the binding of J chain to Ig. In this review, we discuss the roles of pIgR in transcytosis of IgA and IgM, the roles of J chain in the formation of polymeric IgA and IgM and recognition by pIgR, and focus particularly on recent progress in understanding the roles of MZB1, a molecular chaperone protein.
Keywords: antibody secretion; immunoglobulin A (IgA); immunoglobulin M (IgM); immunoglobulin transcytosis; joining chain (J chain); marginal zone B and B1 cell-specific protein (MZB1); mucosal immunity; polymeric immunoglobulin receptor (pIgR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Van Anken E., Pena F., Hafkemeijer N., Christis C., Romijn E.P., Grauschopf U., Oorschot V.M.J., Pertel T., Engels S., Ora A., et al. Efficient IgM Assembly and Secretion Require the Plasma Cell Induced Endoplasmic Reticulum Protein PERp1. Proc. Natl. Acad. Sci. USA. 2009;106:17019–17024. doi: 10.1073/pnas.0903036106. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

