Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents
- PMID: 33669046
- PMCID: PMC7956488
- DOI: 10.3390/ijms22052297
Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents
Abstract
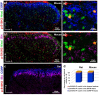
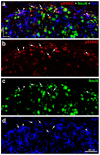
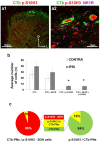
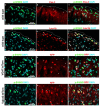
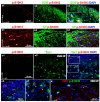
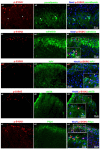
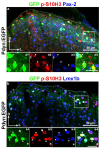
The phosphorylation of serine 10 in histone 3 (p-S10H3) has recently been demonstrated to participate in spinal nociceptive processing. However, superficial dorsal horn (SDH) neurons involved in p-S10H3-mediated nociception have not been fully characterized. In the present work, we combined immunohistochemistry, in situ hybridization with the retrograde labeling of projection neurons to reveal the subset of dorsal horn neurons presenting an elevated level of p-S10H3 in response to noxious heat (60 °C), causing burn injury. Projection neurons only represented a small percentage (5%) of p-S10H3-positive cells, while the greater part of them belonged to excitatory SDH interneurons. The combined immunolabeling of p-S10H3 with markers of already established interneuronal classes of the SDH revealed that the largest subset of neurons with burn injury-induced p-S10H3 expression was dynorphin immunopositive in mice. Furthermore, the majority of p-S10H3-expressing dynorphinergic neurons proved to be excitatory, as they lacked Pax-2 and showed Lmx1b-immunopositivity. Thus, we showed that neurochemically heterogeneous SDH neurons exhibit the upregulation of p-S10H3 shortly after noxious heat-induced burn injury and consequential tissue damage, and that a dedicated subset of excitatory dynorphinergic neurons is likely a key player in the development of central sensitization via the p-S10H3 mediated pathway.
Keywords: burn injury; epigenetic modification; histone; neuropeptides; nociception; superficial dorsal horn neuron.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Sahbaie P., Liang D.Y., Shi X.Y., Sun Y., Clark J.D. Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure. Mol. Pain. 2016;12:1744806916641950. doi: 10.1177/1744806916641950. - DOI - PMC - PubMed
-
- Torres-Perez J.V., Santha P., Varga A., Szucs P., Sousa-Valente J., Gaal B., Sivado M., Andreou A.P., Beattie S., Nagy B., et al. Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain. Sci. Rep. 2017;7:41221. doi: 10.1038/srep41221. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FK125035/National Research, Development and Innovation Office
- ÚNKP-18-4-DE-70/New National Excelence Program of the Ministry of Human Capacities
- ÚNKP-19-4-DE-3/New National Excelence Program of the Ministry of Human Capacities
- ÚNKP-17-3/I/New National Excelence Program of the Ministry of Human Capacities
- KTIA_NAP_13-2-2014-0005/Hungarian Brain Research Program
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

