Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies
- PMID: 33669054
- PMCID: PMC7956496
- DOI: 10.3390/molecules26051232
Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies
Abstract
Despite the virulence and high fatality of coronavirus disease 2019 (COVID-19), no specific antiviral treatment exists until the current moment. Natural agents with immune-promoting potentials such as bee products are being explored as possible treatments. Bee honey and propolis are rich in bioactive compounds that express strong antimicrobial, bactericidal, antiviral, anti-inflammatory, immunomodulatory, and antioxidant activities. This review examined the literature for the anti-COVID-19 effects of bee honey and propolis, with the aim of optimizing the use of these handy products as prophylactic or adjuvant treatments for people infected with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Molecular simulations show that flavonoids in propolis and honey (e.g., rutin, naringin, caffeic acid phenyl ester, luteolin, and artepillin C) may inhibit viral spike fusion in host cells, viral-host interactions that trigger the cytokine storm, and viral replication. Similar to the potent antiviral drug remdesivir, rutin, propolis ethanolic extract, and propolis liposomes inhibited non-structural proteins of SARS-CoV-2 in vitro, and these compounds along with naringin inhibited SARS-CoV-2 infection in Vero E6 cells. Propolis extracts delivered by nanocarriers exhibit better antiviral effects against SARS-CoV-2 than ethanolic extracts. In line, hospitalized COVID-19 patients receiving green Brazilian propolis or a combination of honey and Nigella sativa exhibited earlier viral clearance, symptom recovery, discharge from the hospital as well as less mortality than counterparts receiving standard care alone. Thus, the use of bee products as an adjuvant treatment for COVID-19 may produce beneficial effects. Implications for treatment outcomes and issues to be considered in future studies are discussed.
Keywords: ACE-II; COVID -19; SARS-CoV-2; bee honey; bee products; coronavirus disease 2019; coronaviruses; cytokine storm; flavonoids; in silico; in vitro; main protease; molecular docking/biochemical modeling; non-structural proteins; propolis; randomized clinical trials; severe acute respiratory syndrome; spike glycoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
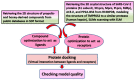
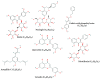
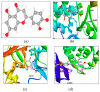

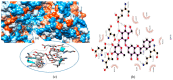
References
-
- Maaroufi H. The Spike Protein S1 Subunit of SARS-CoV-2 Contains an LxxIxE-like Motif that is Known to Recruit the Host PP2A-B56 Phosphatase. bioRxiv. 2020:020941. doi: 10.1101/2020.04.01.020941. - DOI
-
- Kumar V., Dhanjal J.K., Bhargava P., Kaul A., Wang J., Zhang H., Kaul S.C., Wadhwa R., Sundar D. Withanone and withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1775704. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

