Descriptive Histopathological and Ultrastructural Study of Hepatocellular Alterations Induced by Aflatoxin B1 in Rats
- PMID: 33669202
- PMCID: PMC7919794
- DOI: 10.3390/ani11020509
Descriptive Histopathological and Ultrastructural Study of Hepatocellular Alterations Induced by Aflatoxin B1 in Rats
Abstract
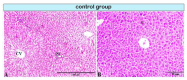
Liver sinusoids are lined by fenestrated endothelial cells surrounded by perisinusoidal cells, Kupffer cells, and pit cells, as well as large granular lymphocytes. The functional ability of the liver cells can be substantially modified by exposure to toxins. In the current work, we assessed the histopathological and ultrastructural effects of a time-course exposure to aflatoxin B1 (AFB1) on the hepatic structures of rats. A total of 30 adult female Wistar rats were randomly divided into three groups: a control group, a group orally administered 250 µg/kg body weight/day of AFB1 for 5 days/week over 4 weeks, and a group that received the same AFB1 treatment but over 8 weeks. Histopathological and ultrastructural examinations of hepatocytes revealed massive vacuolar degeneration and signs of necrosis. Furthermore, the rat liver of the treated group exhibited damage to the sinusoidal endothelium, invasion of the space of Disse with hyperactive Kupffer cells, and some immune cells, as well as Ito cells overloaded with lipids. In addition, damaged telocytes were observed. Taken together, our results indicate that AFB1 induces irreversible adverse effects on the livers of rats.
Keywords: Ito cells; Kupffer cells; aflatoxin B1; fibrosis; necrosis; ultrastructure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Sana S., Anjum A.A., Yaqub T., Nasir M., Ali M.A., Abbas M. Molecular Approaches for Characterization of Aflatoxin Producing Aspergillus flavus Isolates from Poultry Feed. Pak. Vet. J. 2019;39:169–174. doi: 10.29261/pakvetj/2019.031. - DOI
-
- Wu F. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 2007;137:363–374. doi: 10.1016/j.anifeedsci.2007.06.010. - DOI
-
- Pankaj S., Shi H., Keener K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018;71:73–83. doi: 10.1016/j.tifs.2017.11.007. - DOI
-
- Basappa S. Aflatoxins: Formation, Analysis and Control. Alpha Science International; Oxford, UK: 2009.
LinkOut - more resources
Full Text Sources
Other Literature Sources

