Pathology and Classification of SCLC
- PMID: 33669241
- PMCID: PMC7919820
- DOI: 10.3390/cancers13040820
Pathology and Classification of SCLC
Abstract
Lung cancer is consistently the leading cause of cancer-related death worldwide, and it ranks as the second most frequent type of new cancer cases diagnosed in the United States, both in males and females. One subtype of lung cancer, small cell lung carcinoma (SCLC), is an aggressive, poorly differentiated, and high-grade neuroendocrine carcinoma that accounts for 13% of all lung carcinomas. SCLC is the most frequent neuroendocrine lung tumor, and it is commonly presented as an advanced stage disease in heavy smokers. Due to its clinical presentation, it is typically diagnosed in small biopsies or cytology specimens, with routine immunostaining only. However, immunohistochemistry markers are extremely valuable in demonstrating neuroendocrine features of SCLC and supporting its differential diagnosis. The 2015 WHO classification grouped all pulmonary neuroendocrine carcinomas in one category and maintained the SCLC combined variant that was previously recognized. In this review, we explore multiple aspects of the pathologic features of this entity, as well as clinically relevant immunohistochemistry markers expression and its molecular characteristics. In addition, we will focus on characteristics of the tumor microenvironment, and the latest pathogenesis findings to better understand the new therapeutic options in the current era of personalized therapy.
Keywords: biology of SCLC; immune-checkpoint inhibitors in SCLC; pathology and classification of SCLC.
Conflict of interest statement
I.I.W. receives research funding from Genentech, Oncoplex, HTG Molecular, DepArray, Merck, Bristol-Myers Squibb, Medimmune, Adaptive, Adaptimmune, EMD Serono, Pfizer, Takeda, Amgen, Karus, Johnson & Johnson, Bayer, Iovance, 4D, Novartis, and Akoya. I.I.W. sits on the advisory board of the following companies: Genentech/Roche, Bayer, Bristol-Myers Squibb, Astra Zeneca/Medimmune, Pfizer, HTG Molecular, Asuragen, Merck, GlaxoSmithKline, Guardant Health, Oncocyte, and MSD. The funders had no role in the writing of the manuscript.
Figures
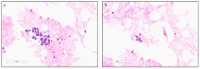
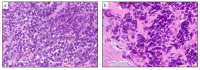

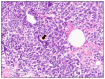
References
-
- International Agency for Research on Cancer (WHO) [(accessed on 11 December 2020)]; Available online: https://gco.iarc.fr/today/home.
-
- American Cancer Society Key Statistics for Small Cell Lung Cancer. [(accessed on 11 December 2020)]; Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html.
-
- National Health Institute Surveillance, Edipemiology, and End Results Program (SEER) [(accessed on 11 December 2020)]; Available online: https://seer.cancer.gov/statfacts/html/lungb.html.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

