Nerve Growth Factor Neutralization Promotes Oligodendrogenesis by Increasing miR-219a-5p Levels
- PMID: 33669304
- PMCID: PMC7920049
- DOI: 10.3390/cells10020405
Nerve Growth Factor Neutralization Promotes Oligodendrogenesis by Increasing miR-219a-5p Levels
Abstract
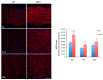
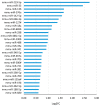
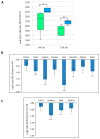
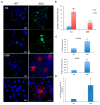
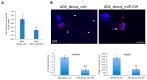
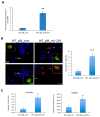
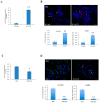
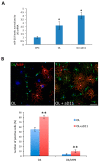
In the brain, the neurotrophin Nerve growth factor (NGF) regulates not only neuronal survival and differentiation, but also glial and microglial functions and neuroinflammation. NGF is known to regulate oligodendrogenesis, reducing myelination in the central nervous system (CNS). In this study, we found that NGF controls oligodendrogenesis by modulating the levels of miR-219a-5p, a well-known positive regulator of oligodendrocyte differentiation. We exploited an NGF-deprivation mouse model, the AD11 mice, in which the postnatal expression of an anti-NGF antibody leads to NGF neutralization and progressive neurodegeneration. Notably, we found that these mice also display increased myelination. A microRNA profiling of AD11 brain samples and qRT-PCR analyses revealed that NGF deprivation leads to an increase of miR-219a-5p levels in hippocampus and cortex and a corresponding down-regulation of its predicted targets. Neurospheres isolated from the hippocampus of AD11 mice give rise to more oligodendrocytes and this process is dependent on miR-219a-5p, as shown by decoy-mediated inhibition of this microRNA. Moreover, treatment of AD11 neurospheres with NGF inhibits miR-219a-5p up-regulation and, consequently, oligodendrocyte differentiation, while anti-NGF treatment of wild type (WT) oligodendrocyte progenitors increases miR-219a-5p expression and the number of mature cells. Overall, this study indicates that NGF inhibits oligodendrogenesis and myelination by down-regulating miR-219a-5p levels, suggesting a novel molecular circuitry that can be exploited for the discovery of new effectors for remyelination in human demyelinating diseases, such as Multiple Sclerosis.
Keywords: Nerve growth factor (NGF); demyelinating diseases; miR-219a-5p; microRNAs; myelin; oligodendrogenesis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Mei F., Lehmann-Horn K., Shen Y.A., Rankin K.A., Stebbins K.J., Lorrain D.S., Pekarek K., Sagan S.A., Xiao L., Teuscher C., et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife. 2016;5:e18246. doi: 10.7554/eLife.18246. - DOI - PMC - PubMed
-
- Zawadzka M., Rivers L.E., Fancy S.P., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M., et al. CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as well as Oligodendrocytes during Repair of CNS Demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

