Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport
- PMID: 33669306
- PMCID: PMC7920073
- DOI: 10.3390/pharmaceutics13020267
Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport
Abstract
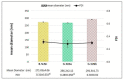
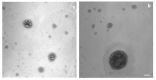
Genistein (GEN) is a soy-derived isoflavone that exhibits several biological effects, such as neuroprotective activity and the prevention of several types of cancer and cardiovascular disease. However, due to its poor water solubility and the extensive first-pass metabolism, the oral bioavailability of GEN is limited. In this work, solid lipid nanoparticles (SLN) were developed to preferentially reach the intestinal lymphatic vessels, avoiding the first-pass metabolism of GEN. GEN-loaded SLN were obtained by a hot homogenization process, and the formulation parameters were chosen based on already formulated studies. The nanoparticles were characterized, and the preliminary in vitro chylomicron formation was evaluated. The cell uptake of selected nanocarriers was studied on the Caco-2 cell line and intestinal mucosa. The SLN, characterized by a spherical shape, showed an average diameter (about 280 nm) suitable for an intestinal lymphatic uptake, good stability during the testing time, and high drug loading capacity. Furthermore, the intestinal mucosa and Caco-2 cells were found to uptake SLN. The approximately two-fold increase in particle size suggested a possible interaction between SLN and the lipid components of chylomicrons like phospholipid; therefore, the results may support the potential for these SLN to improve oral GEN bioavailability via intestinal lymphatic absorption.
Keywords: genistein; intestinal lymphatic absorption; oral bioavailability; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Solid Lipid Nanoparticles of Dronedarone Hydrochloride for Oral Delivery: Optimization, In Vivo Pharmacokinetics and Uptake Studies.Pharm Nanotechnol. 2019;7(5):375-388. doi: 10.2174/2211738507666190802140607. Pharm Nanotechnol. 2019. PMID: 31376827
-
2-Monoacylglycerol Mimetic Liposomes to Promote Intestinal Lymphatic Transport for Improving Oral Bioavailability of Dihydroartemisinin.Int J Nanomedicine. 2024 Jun 6;19:5273-5295. doi: 10.2147/IJN.S462374. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38859952 Free PMC article.
-
Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach.Int J Pharm. 2015 Nov 10;495(1):439-446. doi: 10.1016/j.ijpharm.2015.09.014. Epub 2015 Sep 11. Int J Pharm. 2015. PMID: 26367780
-
Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs.Expert Opin Drug Deliv. 2018 Aug;15(8):787-804. doi: 10.1080/17425247.2018.1503249. Epub 2018 Jul 26. Expert Opin Drug Deliv. 2018. PMID: 30025212 Review.
-
Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability.AAPS PharmSciTech. 2019 Feb 25;20(3):121. doi: 10.1208/s12249-019-1337-8. AAPS PharmSciTech. 2019. PMID: 30805893 Review.
Cited by
-
Nanotechnologies for Physiology-Informed Drug Delivery to the Lymphatic System.Annu Rev Biomed Eng. 2023 Jun 8;25:233-256. doi: 10.1146/annurev-bioeng-092222-034906. Epub 2023 Mar 31. Annu Rev Biomed Eng. 2023. PMID: 37000965 Free PMC article. Review.
-
Improving in vivo oral bioavailability of a poorly soluble drug: a case study on polymeric versus lipid nanoparticles.Drug Deliv Transl Res. 2023 Apr;13(4):1128-1139. doi: 10.1007/s13346-022-01278-4. Epub 2022 Dec 12. Drug Deliv Transl Res. 2023. PMID: 36509967
-
Development of phospholipon®90H complex nanocarrier with enhanced oral bioavailability and anti-inflammatory potential of genistein.Drug Deliv. 2023 Dec;30(1):2162158. doi: 10.1080/10717544.2022.2162158. Drug Deliv. 2023. PMID: 36587626 Free PMC article.
-
Solid Dispersions of Genistein via Solvent Rotary Evaporation for Improving Solubility, Bioavailability, and Amelioration Effect in HFD-Induced Obesity Mice.Pharmaceutics. 2024 Feb 22;16(3):306. doi: 10.3390/pharmaceutics16030306. Pharmaceutics. 2024. PMID: 38543200 Free PMC article.
-
Plant-Derived Anti-Cancer Therapeutics and Biopharmaceuticals.Bioengineering (Basel). 2024 Dec 25;12(1):7. doi: 10.3390/bioengineering12010007. Bioengineering (Basel). 2024. PMID: 39851281 Free PMC article. Review.
References
-
- Mishra A., Vuddanda P.R., Singh S. Intestinal Lymphatic Delivery of Praziquantel by Solid Lipid Nanoparticles: Formulation Design, In Vitro and In Vivo Studies. J. Nanotechnol. 2014;2014:1–12. doi: 10.1155/2014/351693. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

