Anti-Inflammatory, Antidiabetic Properties and In Silico Modeling of Cucurbitane-Type Triterpene Glycosides from Fruits of an Indian Cultivar of Momordica charantia L
- PMID: 33669312
- PMCID: PMC7920048
- DOI: 10.3390/molecules26041038
Anti-Inflammatory, Antidiabetic Properties and In Silico Modeling of Cucurbitane-Type Triterpene Glycosides from Fruits of an Indian Cultivar of Momordica charantia L
Abstract
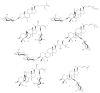
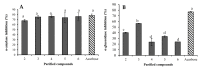
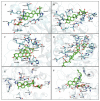
Diabetes mellitus is a chronic disease and one of the fastest-growing health challenges of the last decades. Studies have shown that chronic low-grade inflammation and activation of the innate immune system are intimately involved in type 2 diabetes pathogenesis. Momordica charantia L. fruits are used in traditional medicine to manage diabetes. Herein, we report the purification of a new 23-O-β-d-allopyranosyl-5β,19-epoxycucurbitane-6,24-diene triterpene (charantoside XV, 6) along with 25ξ-isopropenylchole-5(6)-ene-3-O-β-d-glucopyranoside (1), karaviloside VI (2), karaviloside VIII (3), momordicoside L (4), momordicoside A (5) and kuguaglycoside C (7) from an Indian cultivar of Momordica charantia. At 50 µM compounds, 2-6 differentially affected the expression of pro-inflammatory markers IL-6, TNF-α, and iNOS, and mitochondrial marker COX-2. Compounds tested for the inhibition of α-amylase and α-glucosidase enzymes at 0.87 mM and 1.33 mM, respectively. Compounds showed similar α-amylase inhibitory activity than acarbose (0.13 mM) of control (68.0-76.6%). Karaviloside VIII (56.5%) was the most active compound in the α-glucosidase assay, followed by karaviloside VI (40.3%), while momordicoside L (23.7%), A (33.5%), and charantoside XV (23.9%) were the least active compounds. To better understand the mode of binding of cucurbitane-triterpenes to these enzymes, in silico docking of the isolated compounds was evaluated with α-amylase and α-glucosidase.
Keywords: Momordica charantia; anti-inflammatory activity; charantoside XV; cucurbitane-type triterpene glycosides; in silico study; α-amylase; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bharathi L.K., John K.J. Momordica Genus in Asia—An Overview. Springer; New Delhi, India: 2013.
-
- Snee L.S., Nerurkar V.R., Dooley D.A., Efird J.T., Shovic A.C., Nerurkar P.V. Strategies to improve palatability and increase consumption intentions for Momordica charantia (bitter melon): A vegetable commonly used for diabetes management. Nutr. J. 2011;10:78–88. doi: 10.1186/1475-2891-10-78. - DOI - PMC - PubMed
-
- Tan M.-J., Ye J.-M., Turner N., Hohnen-Behrens C., Ke C.-Q., Tang C.-P., Chen T., Weiss H.-C., Gesing E.-R., Rowland A. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem. Biol. 2008;15:263–273. doi: 10.1016/j.chembiol.2008.01.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

