Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells
- PMID: 33669332
- PMCID: PMC7920308
- DOI: 10.3390/nano11020497
Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells
Abstract
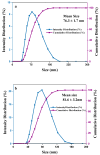
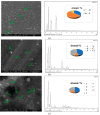
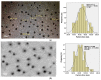
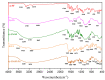
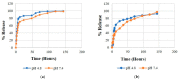
The formation of two nanodelivery systems, Sorafenib (SF)-loaded chitosan (SF-CS) and their folate-coated (SF-CS-FA) nanoparticles (NPs), were developed to enhance SF drug delivery on human Hepatocellular Carcinoma (HepG2) and Colorectal Adenocarcinoma (HT29) cell lines. The ionic gelation method was adopted to synthesize the NPs. The characterizations were performed by DLS, FESEM, TEM, XRD, TGA, FTIR, and UV-visible spectroscopy. It was found that 83.7 ± 2.4% and 87.9 ± 1.1% of encapsulation efficiency; 18.2 ± 1.3% and 19.9 ± 1.4% of loading content; 76.3 ± 13.7 nm and 81.6 ± 12.9 nm of hydrodynamic size; 60-80 nm and 70-100 nm of TEM; and FESEM sizes of near-spherical shape were observed, respectively, for SF-CS and SF-CS-FA nanoparticles. The SF showed excellent release from the nanoparticles under pH 4.8 PBS solution, indicating a good delivery system for tumor cells. The cytotoxicity study revealed their better anticancer action towards HepG2 and HT29 cell lines compared to the free sorafenib. Moreover, both NPs systems showed negligible toxicity to normal Human Dermal Fibroblast adult cells (HDFa). This is towards an enhanced anticancer drug delivery system with sustained-release properties for better cancer management.
Keywords: HDFa; HT29; HepG2; Sorafenib; cancer; cell lines; chitosan-nanoparticles; drug-delivery; folic acid; therapeutic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
LinkOut - more resources
Full Text Sources
Other Literature Sources

