Emerging Immunotherapy for Acute Myeloid Leukemia
- PMID: 33669431
- PMCID: PMC7920435
- DOI: 10.3390/ijms22041944
Emerging Immunotherapy for Acute Myeloid Leukemia
Abstract
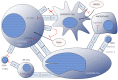
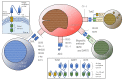
Several immune checkpoint molecules and immune targets in leukemic cells have been investigated. Recent studies have suggested the potential clinical benefits of immuno-oncology (IO) therapy against acute myeloid leukemia (AML), especially targeting CD33, CD123, and CLL-1, as well as immune checkpoint inhibitors (e.g., anti-PD (programmed cell death)-1 and anti-CTLA4 (cytotoxic T-lymphocyte-associated protein 4) antibodies) with or without conventional chemotherapy. Early-phase clinical trials of chimeric antigen receptor (CAR)-T or natural killer (NK) cells for relapsed/refractory AML showed complete remission (CR) or marked reduction of marrow blasts in a few enrolled patients. Bi-/tri-specific antibodies (e.g., bispecific T-cell engager (BiTE) and dual-affinity retargeting (DART)) exhibited 11-67% CR rates with 13-78% risk of cytokine-releasing syndrome (CRS). Conventional chemotherapy in combination with anti-PD-1/anti-CTLA4 antibody for relapsed/refractory AML showed 10-36% CR rates with 7-24 month-long median survival. The current advantages of IO therapy in the field of AML are summarized herein. However, although cancer vaccination should be included in the concept of IO therapy, it is not mentioned in this review because of the paucity of relevant evidence.
Keywords: acute myeloid leukemia (AML); bispecific T-cell engager (BiTE); chimeric antigen receptor (CAR); dual-affinity retargeting (DART); immune check-point inhibitor (ICI); trispecific killer cell engager (TriKE).
Conflict of interest statement
Y.M. received research funding from Ono and CMIC, and honoraria from Bristol-Myers Squibb, Novartis, Astellas, and Daiichi-Sankyo. The other authors declare no conflict of interest.
Figures
References
-
- Goebeler M.-E., Knop S., Viardot A., Kufer P., Topp M.S., Einsele H., Noppeney R., Hess G., Kallert S., Mackensen A., et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J. Clin. Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. - DOI - PubMed
-
- Viardot A., Goebeler M.-E., Hess G., Neumann S., Pfreundschuh M., Adrian N., Zettl F., Libicher M., Sayehli C., Stieglmaier J., et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

