Ym155 Induces Oxidative Stress-Mediated DNA Damage and Cell Cycle Arrest, and Causes Programmed Cell Death in Anaplastic Thyroid Cancer Cells
- PMID: 33669447
- PMCID: PMC7920419
- DOI: 10.3390/ijms22041961
Ym155 Induces Oxidative Stress-Mediated DNA Damage and Cell Cycle Arrest, and Causes Programmed Cell Death in Anaplastic Thyroid Cancer Cells
Abstract
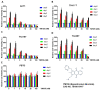
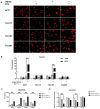
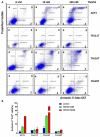
Anaplastic thyroid cancer (ATC) is one of the most lethal malignancies with a median survival time of about 4 months. Currently, there is no effective treatment, and the development of new therapies is an important and urgent issue for ATC patients. YM155 is a small molecule that was identified as the top candidate in a high-throughput screen of small molecule inhibitors performed against a panel of ATC cell lines by the National Cancer Institute. However, there were no follow-up studies investigating YM155 in ATC. Here, we determined the effects of YM155 on ATC and human primary benign thyroid cell (PBTC) survival with alamarBlue assay. Our data show that YM155 inhibited proliferation of ATC cell lines while sparing normal thyroid cells, suggesting a high therapeutic window. YM155-induced DNA damage was detected by measuring phosphorylation of γ-H2AX as a marker for DNA double-strand breaks. The formamidopyrimidine-DNA glycosylase (FPG)-modified alkaline comet assay in conjunction with reactive oxygen species (ROS) assay and glutathione (GSH)/glutathione (GSSG) assay suggests that YM155-mediated oxidative stress contributes to DNA damage. In addition, we provide evidence that YM155 causes cell cycle arrest in S phase and in the G2/M transition and causes apoptosis, as seen with flow cytometry. In this study, we show for the first time the multiple effects of YM155 in ATC cells, furthering a potential therapeutic approach for ATC.
Keywords: DNA damage; YM155; anaplastic thyroid cancer; apoptosis; cell cycle arrest.
Conflict of interest statement
Paul M. Weinberger is chief executive officer for SpheroFill LLC, which has no competing interest or relationship with the current research. The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

