Recent Advances in the Chemical Biology of N-Glycans
- PMID: 33669465
- PMCID: PMC7920464
- DOI: 10.3390/molecules26041040
Recent Advances in the Chemical Biology of N-Glycans
Abstract
Asparagine-linked N-glycans on proteins have diverse structures, and their functions vary according to their structures. In recent years, it has become possible to obtain high quantities of N-glycans via isolation and chemical/enzymatic/chemoenzymatic synthesis. This has allowed for progress in the elucidation of N-glycan functions at the molecular level. Interaction analyses with lectins by glycan arrays or nuclear magnetic resonance (NMR) using various N-glycans have revealed the molecular basis for the recognition of complex structures of N-glycans. Preparation of proteins modified with homogeneous N-glycans revealed the influence of N-glycan modifications on protein functions. Furthermore, N-glycans have potential applications in drug development. This review discusses recent advances in the chemical biology of N-glycans.
Keywords: N-glycan; NMR; chemical biology; glycan array; glycoprotein; lectin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

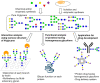

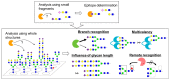
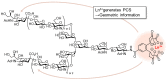
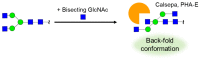

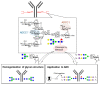
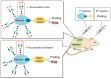
References
-
- Wu B., Hua Z., Warren J.D., Ranganathan K., Wan Q., Chen G., Tan Z., Chen J., Endo A., Danishefsky S.J. Synthesis of the fucosylated biantennary N-glycan of erythropoietin. Tetrahedron Lett. 2006;47:5577–5579. doi: 10.1016/j.tetlet.2006.05.086. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

