Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens
- PMID: 33669483
- PMCID: PMC7922508
- DOI: 10.3390/cells10020438
Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens
Abstract
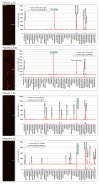
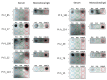
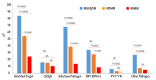
Chronic stimulation by infectious pathogens or self-antigen glucosylsphingosine (GlcSph) can lead to monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM). Novel assays such as the multiplex infectious antigen microarray (MIAA) and GlcSph assays, permit identification of targets for >60% purified monoclonal immunoglobulins (Igs). Searching for additional targets, we selected 28 purified monoclonal Igs whose antigen was not represented on the MIAA and GlcSph assays; their specificity of recognition was then analyzed using microarrays consisting of 3760 B-cell epitopes from 196 pathogens. The peptide sequences PALTAVETG and PALTAAETG of the VP1 coat proteins of human poliovirus 1/3 and coxsackievirus B1/B3, respectively, were specifically recognized by 6/28 monoclonal Igs. Re-analysis of patient cohorts showed that purified monoclonal Igs from 10/155 MGUS/SM (6.5%) and 3/147 MM (2.0%) bound to the PALTAVETG or PALTAAETG epitopes. Altogether, PALTAV/AETG-initiated MGUS are not rare and few seem to evolve toward myeloma.
Keywords: MGUS; antigen specificity; coxsackievirus; infection; monoclonal gammopathy; monoclonal immunoglobulin; multiple myeloma; pathogen; poliovirus.
Conflict of interest statement
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures


References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

