Endogenous Double-Stranded RNA
- PMID: 33669629
- PMCID: PMC7930956
- DOI: 10.3390/ncrna7010015
Endogenous Double-Stranded RNA
Abstract
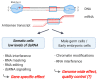
The birth of long non-coding RNAs (lncRNAs) is closely associated with the presence and activation of repetitive elements in the genome. The transcription of endogenous retroviruses as well as long and short interspersed elements is not only essential for evolving lncRNAs but is also a significant source of double-stranded RNA (dsRNA). From an lncRNA-centric point of view, the latter is a minor source of bother in the context of the entire cell; however, dsRNA is an essential threat. A viral infection is associated with cytoplasmic dsRNA, and endogenous RNA hybrids only differ from viral dsRNA by the 5' cap structure. Hence, a multi-layered defense network is in place to protect cells from viral infections but tolerates endogenous dsRNA structures. A first line of defense is established with compartmentalization; whereas endogenous dsRNA is found predominantly confined to the nucleus and the mitochondria, exogenous dsRNA reaches the cytoplasm. Here, various sensor proteins recognize features of dsRNA including the 5' phosphate group of viral RNAs or hybrids with a particular length but not specific nucleotide sequences. The sensors trigger cellular stress pathways and innate immunity via interferon signaling but also induce apoptosis via caspase activation. Because of its central role in viral recognition and immune activation, dsRNA sensing is implicated in autoimmune diseases and used to treat cancer.
Keywords: antisense transcript; double-stranded RNA (dsRNA); innate immunity; repetitive DNA elements (RE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

