Exosomal HMGB1 Promoted Cancer Malignancy
- PMID: 33669632
- PMCID: PMC7921955
- DOI: 10.3390/cancers13040877
Exosomal HMGB1 Promoted Cancer Malignancy
Abstract
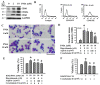
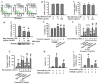
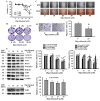
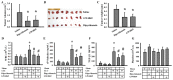
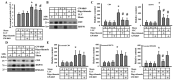
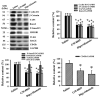
Reciprocal crosstalk between platelets and malignancies underscores the potential of antiplatelet therapy in cancer treatment. In this study, we found that human chronic myeloid leukemia K562 cell-differentiated megakaryocytes and murine platelets produced bioactive substances and these are released into the extracellular space, partly in their exosomal form. High-mobility group box 1 (HMGB1) is a type of exosomal cargo, and the antiplatelet drugs aspirin and dipyridamole interfered with its incorporation into the exosomes. Those released substances and exosomes, along with exogenous HMGB1, promoted cancer cell survival and protected cells from doxorubicin cytotoxicity. In a tumor-bearing model established using murine Lewis lung carcinoma (LLC) cells and C57BL/6 mice, the tumor suppressive effect of dipyridamole correlated well with decreased circulating white blood cells, soluble P-selectin, TGF-β1 (Transforming Growth Factor-β1), exosomes, and exosomal HMGB1, as well as tumor platelet infiltration. Exosome release inhibitor GW4869 exhibited suppressive effects as well. The suppressive effect of dipyridamole on cancer cell survival was paralleled by a reduction of HMGB1/receptor for advanced glycation end-products axis, and proliferation- and migration-related β-catenin, Yes-associated protein 1, Runt-related transcription factor 2, and TGF- β1/Smad signals. Therefore, exosomes and exosomal HMGB1 appear to have roles in platelet-driven cancer malignancy and represent targets of antiplatelet drugs in anticancer treatment.
Keywords: HMGB1; antiplatelet; exosome; malignancy.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Zhao J.M., Wang Y.H., Yao N., Wei K.K., Jiang L., Hanif S., Wang Z.X. Poor prognosis significance of pretreatment thrombocytosis in patients with colorectal cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2016;17:4295–4300. - PubMed
-
- Mezouar S., Darbousset R., Dignat-George F., Panicot-Dubois L., Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int. J. Cancer. 2015;136:462–475. doi: 10.1002/ijc.28997. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

