Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy
- PMID: 33669817
- PMCID: PMC7922180
- DOI: 10.3390/molecules26041109
Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy
Abstract
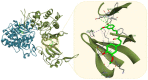
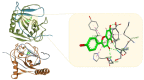
Despite the recent advances in the field of chemically synthetized pharmaceutical agents, nature remains the main supplier of bioactive molecules. The research of natural products is a valuable approach for the discovery and development of novel biologically active compounds possessing unique structures and mechanisms of action. Although their use belongs to the traditional treatment regimes, plant-derived compounds still cover a large portion of the current-day pharmaceutical agents. Their medical importance is well recognized in the field of oncology, especially as an alternative to the limitations of conventional chemotherapy (severe side effects and inefficacy due to the occurrence of multi-drug resistance). This review offers a comprehensive perspective of the first blockbuster chemotherapeutic agents of natural origin's (e.g. taxol, vincristine, doxorubicin) mechanism of action using 3D representation. In addition is portrayed the step-by-step evolution from preclinical to clinical evaluation of the most recently studied natural compounds with potent antitumor activity (e.g. resveratrol, curcumin, betulinic acid, etc.) in terms of anticancer mechanisms of action and the possible indications as chemotherapeutic or chemopreventive agents and sensitizers. Finally, this review describes several efficient platforms for the encapsulation and targeted delivery of natural compounds in cancer treatment.
Keywords: bioactive compounds; chemoprevention; doxorubicin, paclitaxel, vincristine, resveratrol, curcumin, rutin, betulinic acid, antitumoral effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
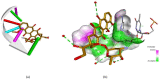


References
-
- Koparde A.A., Doijad R.C., Magdum C.S. Natural Products in Drug Discovery. [(accessed on 16 November 2020)]; Available online: https://www.intechopen.com/books/pharmacognosy-medicinal-plants/natural-....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

