Human TRIM5α: Autophagy Connects Cell-Intrinsic HIV-1 Restriction and Innate Immune Sensor Functioning
- PMID: 33669846
- PMCID: PMC7923229
- DOI: 10.3390/v13020320
Human TRIM5α: Autophagy Connects Cell-Intrinsic HIV-1 Restriction and Innate Immune Sensor Functioning
Abstract
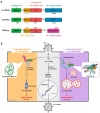
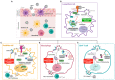
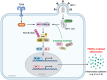
Human immunodeficiency virus-1 (HIV-1) persists as a global health concern, with an incidence rate of approximately 2 million, and estimated global prevalence of over 35 million. Combination antiretroviral treatment is highly effective, but HIV-1 patients that have been treated still suffer from chronic inflammation and residual viral replication. It is therefore paramount to identify therapeutically efficacious strategies to eradicate viral reservoirs and ultimately develop a cure for HIV-1. It has been long accepted that the restriction factor tripartite motif protein 5 isoform alpha (TRIM5α) restricts HIV-1 infection in a species-specific manner, with rhesus macaque TRIM5α strongly restricting HIV-1, and human TRIM5α having a minimal restriction capacity. However, several recent studies underscore human TRIM5α as a cell-dependent HIV-1 restriction factor. Here, we present an overview of the latest research on human TRIM5α and propose a novel conceptualization of TRIM5α as a restriction factor with a varied portfolio of antiviral functions, including mediating HIV-1 degradation through autophagy- and proteasome-mediated mechanisms, and acting as a viral sensor and effector of antiviral signaling. We have also expanded on the protective antiviral roles of autophagy and outline the therapeutic potential of autophagy modulation to intervene in chronic HIV-1 infection.
Keywords: CD4+ T cells; HIV-1 restriction; Langerhans cells; Langerin; TRIM5α; antiviral immunity; autophagy; dendritic cells; macrophages; viral evasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization HIV/AIDS Factsheets. [(accessed on 19 October 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
-
- Hammer S.M., Katzenstein D.A., Hughes M.D., Gundacker H., Schooley R.T., Haubrich R.H., Henry W.K., Lederman M.M., Phair J.P., Niu M.T., et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N. Engl. J. Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. - DOI - PubMed
-
- Egger M., Hirschel B., Francioli P., Sudre P., Wirz M., Flepp M., Rickenbach M., Malinverni R., Vernazza P., Battegay M. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: Prospective multicentre study. Swiss HIV cohort study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

