Synthesis and Biological Evaluation of Novel 4-(4-Formamidophenylamino)- N-methylpicolinamide Derivatives as Potential Antitumor Agents
- PMID: 33670007
- PMCID: PMC7926825
- DOI: 10.3390/molecules26041150
Synthesis and Biological Evaluation of Novel 4-(4-Formamidophenylamino)- N-methylpicolinamide Derivatives as Potential Antitumor Agents
Abstract
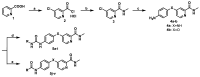
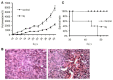
A novel series of 4-(4-formamidophenylamino)-N-methylpicolinamide derivatives were synthesized and evaluated against different tumor cell lines. Experiments in vitro showed that these derivatives could inhibit the proliferation of two kinds of human cancer cell lines (HepG2, HCT116) at low micromolar concentrations and the most potent analog 5q possessed broad-spectrum antiproliferative activity. Experiments in vivo demonstrated that 5q could effectively prolong the longevity of colon carcinoma-burdened mice and slow down the progression of cancer cells by suppression of angiogenesis and the induction of apoptosis and necrosis.
Keywords: 4-(4-formamidophenylamino)-N-methylpicolinamide derivatives; angiogenesis inhibitors; anti-proliferation; apoptosis; pharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Synthesis and bioevaluation study of novel N-methylpicolinamide and thienopyrimidine derivatives as selectivity c-Met kinase inhibitors.Bioorg Med Chem. 2018 Jan 1;26(1):245-256. doi: 10.1016/j.bmc.2017.11.039. Epub 2017 Nov 26. Bioorg Med Chem. 2018. PMID: 29203143
-
Synthesis and biological evaluation of novel N-methyl-picolinamide-4-thiol derivatives as potential antitumor agents.Molecules. 2012 May 25;17(6):6317-30. doi: 10.3390/molecules17066317. Molecules. 2012. PMID: 22634842 Free PMC article.
-
Design, synthesis and biological evaluation of novel benzimidazole-2-substituted phenyl or pyridine propyl ketene derivatives as antitumour agents.Eur J Med Chem. 2016 May 23;114:328-36. doi: 10.1016/j.ejmech.2016.03.029. Epub 2016 Mar 12. Eur J Med Chem. 2016. PMID: 27017265
-
Design, synthesis, docking, molecular dynamics and bioevaluation studies on novel N-methylpicolinamide and thienopyrimidine derivatives with inhibiting NF-κB and TAK1 activities: Cheminformatics tools RDKit applied in drug design.Eur J Med Chem. 2021 Nov 5;223:113576. doi: 10.1016/j.ejmech.2021.113576. Epub 2021 Jun 5. Eur J Med Chem. 2021. PMID: 34153577
-
Synthesis and biological evaluation of novel 2,3-dihydrochromeno[3,4-d]imidazol-4(1H)-one derivatives as potent anticancer cell proliferation and migration agents.Eur J Med Chem. 2016 May 23;114:232-43. doi: 10.1016/j.ejmech.2016.01.035. Epub 2016 Feb 5. Eur J Med Chem. 2016. PMID: 26994691
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

