Adoptive Immunotherapy beyond CAR T-Cells
- PMID: 33670139
- PMCID: PMC7916861
- DOI: 10.3390/cancers13040743
Adoptive Immunotherapy beyond CAR T-Cells
Abstract
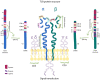
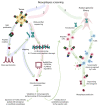
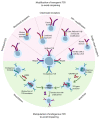
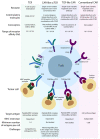
Adoptive cell immunotherapy (ACT) is a vibrant field of cancer treatment that began progressive development in the 1980s. One of the most prominent and promising examples is chimeric antigen receptor (CAR) T-cell immunotherapy for the treatment of B-cell hematologic malignancies. Despite success in the treatment of B-cell lymphomas and leukemia, CAR T-cell therapy remains mostly ineffective for solid tumors. This is due to several reasons, such as the heterogeneity of the cellular composition in solid tumors, the need for directed migration and penetration of CAR T-cells against the pressure gradient in the tumor stroma, and the immunosuppressive microenvironment. To substantially improve the clinical efficacy of ACT against solid tumors, researchers might need to look closer into recent developments in the other branches of adoptive immunotherapy, both traditional and innovative. In this review, we describe the variety of adoptive cell therapies beyond CAR T-cell technology, i.e., exploitation of alternative cell sources with a high therapeutic potential against solid tumors (e.g., CAR M-cells) or aiming to be universal allogeneic (e.g., CAR NK-cells, γδ T-cells), tumor-infiltrating lymphocytes (TILs), and transgenic T-cell receptor (TCR) T-cell immunotherapies. In addition, we discuss the strategies for selection and validation of neoantigens to achieve efficiency and safety. We provide an overview of non-conventional TCRs and CARs, and address the problem of mispairing between the cognate and transgenic TCRs. Finally, we summarize existing and emerging approaches for manufacturing of the therapeutic cell products in traditional, semi-automated and fully automated Point-of-Care (PoC) systems.
Keywords: CAR NK-cell; CAR T-cell; TIL; chimeric antigen receptor; neoantigen; neoepitope; peptide; transgeneic TCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ruella M., Xu J., Barrett D.M., Fraietta J.A., Reich T.J., Ambrose D.E., Klichinsky M., Shestova O., Patel P.R., Kulikovskaya I., et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. - DOI - PMC - PubMed
-
- Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. - DOI - PMC - PubMed
-
- Schmueck-Henneresse M., Omer B., Shum T., Tashiro H., Mamonkin M., Lapteva N., Sharma S., Rollins L., Dotti G., Reinke P., et al. Comprehensive Approach for Identifying the T Cell Subset Origin of CD3 and CD28 Antibody–Activated Chimeric Antigen Receptor–Modified T Cells. J. Immunol. 2017;199:348–362. doi: 10.4049/jimmunol.1601494. - DOI - PMC - PubMed
-
- Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O’Connor R.S., Hwang W.-T.T., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

