Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds
- PMID: 33670141
- PMCID: PMC7916880
- DOI: 10.3390/nano11020456
Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds
Abstract
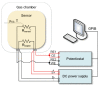
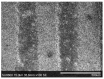
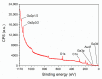
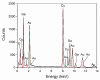
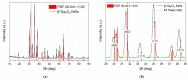
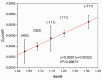
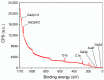
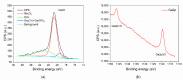
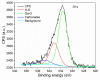
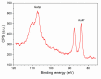
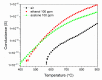
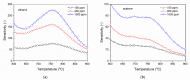
Gas sensitive structures made of nanowires exhibit extremally large specific surface area, and a great number of chemically active centres that can react with the ambient atmosphere. This makes the use of nanomaterials promising for super sensitive gas sensor applications. Monoclinic β-Ga2O3 nanowires (NWs) were synthesized from metallic gallium at atmospheric pressure in the presence of nitrogen and water vapor. The nanowires were grown directly on interdigitated gold electrodes screen printed on Al2O3 substrates, which constituted the gas sensor structure. The observations made with transmission electron microscope (TEM) have shown that the nanowires are monocrystalline and their diameters vary from 80 to 300 nm with the average value of approximately 170 nm. Au droplets were found to be anchored at the tips of the nanowires which may indicate that the nanowires followed the Vapor-Liquid-Solid (VLS) mechanism of growth. The conductivity of β-Ga2O3 NWs increases in the presence of volatile organic compounds (VOC) even in the temperature below 600 °C. The gas sensor based on the synthesized β-Ga2O3 NWs shows peak sensitivity to 100 ppm of ethanol of 75.1 at 760 °C, while peak sensitivity to 100 ppm of acetone is 27.5 at 690 °C.
Keywords: acetone; chemical gas sensors; chemiresistors; ethanol; gallium oxide; metal oxide; nanomaterials; nanowires; thermal synthesis; volatile organic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yamazoe N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005;108:2–14. doi: 10.1016/j.snb.2004.12.075. - DOI
-
- Fiedot-Toboła M., Suchorska-Woźniak P., Startek K., Rac-Rumijowska O., Szukiewicz R., Kwoka M., Teterycz H. Correlation between Microstructure and Chemical Composition of Zinc Oxide Gas Sensor Layers and Their Gas-Sensitive Properties in Chlorine Atmosphere. Sensors. 2020;20:6951. doi: 10.3390/s20236951. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

