Signatures of Dermal Fibroblasts from RDEB Pediatric Patients
- PMID: 33670258
- PMCID: PMC7918539
- DOI: 10.3390/ijms22041792
Signatures of Dermal Fibroblasts from RDEB Pediatric Patients
Abstract
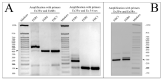
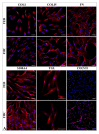
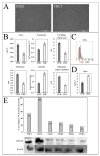
The recessive form of dystrophic epidermolysis bullosa (RDEB) is a debilitating disease caused by impairments in the junctions of the dermis and the basement membrane of the epidermis. Mutations in the COL7A1 gene induce multiple abnormalities, including chronic inflammation and profibrotic changes in the skin. However, the correlations between the specific mutations in COL7A1 and their phenotypic output remain largely unexplored. The mutations in the COL7A1 gene, described here, were found in the DEB register. Among them, two homozygous mutations and two cases of compound heterozygous mutations were identified. We created the panel of primary patient-specific RDEB fibroblast lines (FEB) and compared it with control fibroblasts from healthy donors (FHC). The set of morphological features and the contraction capacity of the cells distinguished FEB from FHC. We also report the relationships between the mutations and several phenotypic traits of the FEB. Based on the analysis of the available RNA-seq data of RDEB fibroblasts, we performed an RT-qPCR gene expression analysis of our cell lines, confirming the differential status of multiple genes while uncovering the new ones. We anticipate that our panels of cell lines will be useful not only for studying RDEB signatures but also for investigating the overall mechanisms involved in disease progression.
Keywords: COL7A1; RDEB; cell culture; dermal fibroblast; differential gene expression; disease mutation; epidermolysis bullosa; extracellular matrix; splicing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Odorisio T., Di Salvio M., Orecchia A., Di Zenzo G., Piccinni E., Cianfarani F., Travaglione A., Uva P., Bellei B., Conti A., et al. Monozygotic Twins Discordant for Recessive Dystrophic Epidermolysis Bullosa Phenotype Highlight the Role of TGF-β Signalling in Modifying Disease Severity. Hum. Mol. Genet. 2014;23:3907–3922. doi: 10.1093/hmg/ddu102. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

