Quantum Dots as a Good Carriers of Unsymmetrical Bisacridines for Modulating Cellular Uptake and the Biological Response in Lung and Colon Cancer Cells
- PMID: 33670297
- PMCID: PMC7917955
- DOI: 10.3390/nano11020462
Quantum Dots as a Good Carriers of Unsymmetrical Bisacridines for Modulating Cellular Uptake and the Biological Response in Lung and Colon Cancer Cells
Abstract
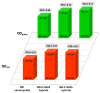
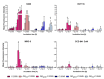
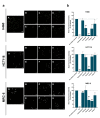
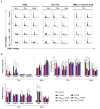
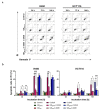
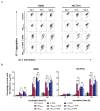
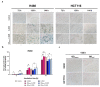
Nanotechnology-based drug delivery provides a promising area for improving the efficacy of cancer treatments. Therefore, we investigate the potential of using quantum dots (QDs) as drug carriers for antitumor unsymmetrical bisacridine derivatives (UAs) to cancer cells. We examine the influence of QD-UA hybrids on the cellular uptake, internalization (Confocal Laser Scanning Microscope), and the biological response (flow cytometry and light microscopy) in lung H460 and colon HCT116 cancer cells. We show the time-dependent cellular uptake of QD-UA hybrids, which were more efficiently retained inside the cells compared to UAs alone, especially in H460 cells, which could be due to multiple endocytosis pathways. In contrast, in HCT116 cells, the hybrids were taken up only by one endocytosis mechanism. Both UAs and their hybrids induced apoptosis in H460 and HCT116 cells (to a greater extent in H460). Cells which did not die underwent senescence more efficiently following QDs-UAs treatment, compared to UAs alone. Cellular senescence was not observed in HCT116 cells following treatment with both UAs and their hybrids. Importantly, QDgreen/red themselves did not provoke toxic responses in cancer or normal cells. In conclusion, QDs are good candidates for targeted UA delivery carriers to cancer cells while protecting normal cells from toxic drug activities.
Keywords: apoptosis; cellular senescence; cellular uptake; internalization; lung and colon cancer cells; quantum dots; unsymmetrical bisacridines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liang B., Li N., Zhang S., Qi A., Feng J., Jing W., Shi C., Ma Z., Gao S. Idarubicin-loaded methoxy poly(ethylene glycol)-b-poly(L-lactide-co-glycolide) nanoparticles for enhancing cellular uptake and promoting antileukemia activity. Int. J. Nanomed. 2019;14:543–556. doi: 10.2147/IJN.S190027. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

