Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades
- PMID: 33670436
- PMCID: PMC7923122
- DOI: 10.3390/molecules26041055
Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades
Abstract
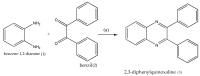
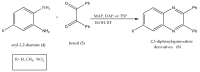
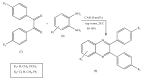
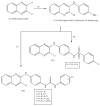
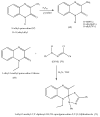
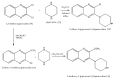
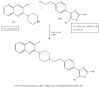
Quinoxalines, a class of N-heterocyclic compounds, are important biological agents, and a significant amount of research activity has been directed towards this class. They have several prominent pharmacological effects like antifungal, antibacterial, antiviral, and antimicrobial. Quinoxaline derivatives have diverse therapeutic uses and have become the crucial component in drugs used to treat cancerous cells, AIDS, plant viruses, schizophrenia, certifying them a great future in medicinal chemistry. Due to the current pandemic situation caused by SARS-COVID 19, it has become essential to synthesize drugs to combat deadly pathogens (bacteria, fungi, viruses) for now and near future. Since quinoxalines is an essential moiety to treat infectious diseases, numerous synthetic routes have been developed by researchers, with a prime focus on green chemistry and cost-effective methods. This review paper highlights the various synthetic routes to prepare quinoxaline and its derivatives, covering the literature for the last two decades. A total of 31 schemes have been explained using the green chemistry approach, cost-effective methods, and quinoxaline derivatives' therapeutic uses.
Keywords: SAR; biological applications; green chemistry; quinoxaline.
Conflict of interest statement
The authors declared no conflict of interest.
Figures












Similar articles
-
Recent Methods for the Synthesis of Quinoxaline Derivatives and their Biological Activities.Mini Rev Med Chem. 2024;24(9):920-982. doi: 10.2174/0113895575264375231012115026. Mini Rev Med Chem. 2024. PMID: 37885112 Review.
-
Quinoxaline Derivatives as Antiviral Agents: A Systematic Review.Molecules. 2020 Jun 16;25(12):2784. doi: 10.3390/molecules25122784. Molecules. 2020. PMID: 32560203 Free PMC article.
-
Quinoxaline-Based Scaffolds Targeting Tyrosine Kinases and Their Potential Anticancer Activity.Arch Pharm (Weinheim). 2016 May;349(5):309-26. doi: 10.1002/ardp.201500468. Epub 2016 Apr 9. Arch Pharm (Weinheim). 2016. PMID: 27062086 Review.
-
Advances in quinoxaline derivatives: synthetic routes and antiviral efficacy against respiratory pathogens.RSC Adv. 2024 Nov 7;14(48):35400-35423. doi: 10.1039/d4ra04292a. eCollection 2024 Nov 4. RSC Adv. 2024. PMID: 39512644 Free PMC article. Review.
-
An insight into medicinal chemistry of anticancer quinoxalines.Bioorg Med Chem. 2019 Jan 1;27(1):16-35. doi: 10.1016/j.bmc.2018.11.021. Epub 2018 Nov 15. Bioorg Med Chem. 2019. PMID: 30502116 Review.
Cited by
-
Advances in Control Strategies against Spodoptera frugiperda. A Review.Molecules. 2021 Sep 15;26(18):5587. doi: 10.3390/molecules26185587. Molecules. 2021. PMID: 34577058 Free PMC article. Review.
-
Photocatalytic Aerobic Dehydrogenation of N-Heterocycles with Ir(III) Photosensitizers Bearing the 2(2'-Pyridyl)benzimidazole Scaffold.Inorg Chem. 2022 Apr 25;61(16):6193-6208. doi: 10.1021/acs.inorgchem.2c00358. Epub 2022 Apr 8. Inorg Chem. 2022. PMID: 35394766 Free PMC article.
-
I2-Catalyzed Carbonylation of α-Methylene Ketones to Synthesize 1,2-Diaryl Diketones and Antiviral Quinoxalines in One Pot.ACS Omega. 2021 Dec 21;7(1):1380-1394. doi: 10.1021/acsomega.1c06017. eCollection 2022 Jan 11. ACS Omega. 2021. PMID: 35036799 Free PMC article.
-
Recent Methods for the Synthesis of Quinoxaline Derivatives and their Biological Activities.Mini Rev Med Chem. 2024;24(9):920-982. doi: 10.2174/0113895575264375231012115026. Mini Rev Med Chem. 2024. PMID: 37885112 Review.
-
Anticancer, anti-inflammatory and analgesic activities of aminoalcohol-based quinoxaline small molecules.Acta Cir Bras. 2024 Aug 5;39:e395124. doi: 10.1590/acb395124. eCollection 2024. Acta Cir Bras. 2024. PMID: 39109780 Free PMC article.
References
-
- Irfan A., Sabeeh I., Umer M., Naqvi A.Z., Fatima H., Yousaf S., Fatima Z. A review on the therapeutic potential of quinoxaline derivatives. World J. Pharm. Res. 2017;6:47–68. doi: 10.20959/wjpr201713-9878. - DOI
-
- Cheeseman G.W.H., Cookson R.F. Chemistry of Heterocyclic Compounds. Volume 35 Wiley-Interscience; Hoboken, NJ, USA: 1979.
-
- Thakuria H., Das G. One-pot efficient green synthesis of 1,4-dihydro-quinoxaline-2,3-dione derivatives. J. Chem. Sci. 2006;118:425–428. doi: 10.1007/BF02711453. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

