Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties
- PMID: 33670448
- PMCID: PMC7922149
- DOI: 10.3390/pharmaceutics13020273
Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties
Abstract
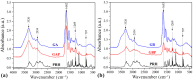
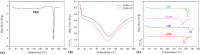
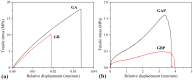
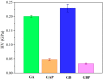
This study processes and characterizes propranolol hydrochloride/gelatin mucoadhesive buccal films. Two types of gelatin are used: Gelatin from porcine skin, type A (GA), and gelatin from bovine skin (GB). The influence of gelatin type on mechanical, mucoadhesive, and biopharmaceutical characteristics of buccal films is evaluated. Fourier-Transfer infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) analysis show that GA with propranolol hydrochloride (PRH) in the film (GAP) formed a physical mixture, whereas GB with PRH (GBP) form a compound-complex. Results of mechanical testing (tensile test, hardness) revealed that GAP films exhibit higher elastic modulus, tensile strength, and hardness. A mucoahesion test shows that GBP has higher adhesion strength, while GAP shows higher work of adhesion. Both in vitro release study and in silico simulation indicated that processed films can provide effective drug transport through the buccal mucosa. In silico simulation shows improved bioavailability from buccal films, in comparison to the immediate-release tablets-indicating that the therapeutic drug dose can be markedly reduced.
Keywords: buccal films; gelatin; in silico simulation; mucoadhesion; propranolol hydrochloride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction.Pharmaceutics. 2021 Dec 13;13(12):2143. doi: 10.3390/pharmaceutics13122143. Pharmaceutics. 2021. PMID: 34959423 Free PMC article.
-
Design and optimization of kollicoat ® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride.Mater Sci Eng C Mater Biol Appl. 2019 Apr;97:230-244. doi: 10.1016/j.msec.2018.12.036. Epub 2018 Dec 12. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30678908
-
Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride.J Pharm Sci. 2015 Dec;104(12):4365-4372. doi: 10.1002/jps.24688. Epub 2015 Oct 27. J Pharm Sci. 2015. PMID: 26505621
-
Manufacture and characterization of mucoadhesive buccal films.Eur J Pharm Biopharm. 2011 Feb;77(2):187-99. doi: 10.1016/j.ejpb.2010.11.023. Epub 2010 Dec 3. Eur J Pharm Biopharm. 2011. PMID: 21130875 Review.
-
Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches.J Control Release. 2022 Dec;352:1071-1092. doi: 10.1016/j.jconrel.2022.10.058. Epub 2022 Nov 18. J Control Release. 2022. PMID: 36351519 Review.
Cited by
-
Electrospun Membranes as a Porous Barrier for Molecular Transport: Membrane Characterization and Release Assessment.Pharmaceutics. 2021 Jun 21;13(6):916. doi: 10.3390/pharmaceutics13060916. Pharmaceutics. 2021. PMID: 34205650 Free PMC article.
-
Drug-Polymers Composite Matrix Tablets: Effect of Hydroxypropyl Methylcellulose (HPMC) K-Series on Porosity, Compatibility, and Release Behavior of the Tablet Containing a BCS Class I Drug.Polymers (Basel). 2022 Aug 19;14(16):3406. doi: 10.3390/polym14163406. Polymers (Basel). 2022. PMID: 36015661 Free PMC article.
-
Enhancing Mucoadhesive Properties of Gelatin through Chemical Modification with Unsaturated Anhydrides.Biomacromolecules. 2024 Mar 11;25(3):1612-1628. doi: 10.1021/acs.biomac.3c01183. Epub 2024 Feb 6. Biomacromolecules. 2024. PMID: 38319691 Free PMC article.
-
In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks.Gels. 2022 May 7;8(5):290. doi: 10.3390/gels8050290. Gels. 2022. PMID: 35621588 Free PMC article.
-
Combination of 3D printing and electrospinning to develop chitin/gelatin/PVA scaffolds.Int J Bioprint. 2023 Mar 6;9(3):701. doi: 10.18063/ijb.701. eCollection 2023. Int J Bioprint. 2023. PMID: 37273981 Free PMC article.
References
-
- Kontogiannidou E., Ferrari M., Deligianni A.-D., Bouropoulos N., Andreadis D.A., Sorrenti M., Catenacci L., Nazari K., Arshad M.S., Chang M.-W., et al. In vitro and ex vivo evaluation of tablets containing piroxicam-cyclodextrin complexes for buccal delivery. Pharmaceutics. 2019;11:398. doi: 10.3390/pharmaceutics11080398. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

