The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.)
- PMID: 33670503
- PMCID: PMC7923115
- DOI: 10.3390/plants10020387
The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.)
Abstract
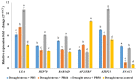
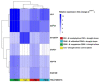
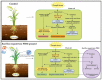
This study assessed the potential of Bacillus endophyticus PB3, Bacillus altitudinis PB46, and Bacillus megaterium PB50 to induce drought tolerance in a susceptible rice cultivar. The leaves of the potted rice plants subjected to physical drought stress for 10 days during the flowering stage were inoculated with single-strain suspensions. Control pots of irrigated and drought-stressed plants were included in the experiment for comparison. In all treatments, the plant stress-related physiochemical and biochemical changes were examined and the expression of six stress-responsive genes in rice leaves was evaluated. The colonization potential on the surface of the rice leaves and stomata of the most successful strain in terms of induced tolerance was confirmed in the gnotobiotic experiment. The plants sprayed with B. megaterium PB50 showed an elevated stress tolerance based on their higher relative water content and increased contents of total sugars, proteins, proline, phenolics, potassium, calcium, abscisic acid, and indole acetic acid, as well as a high expression of stress-related genes (LEA, RAB16B, HSP70, SNAC1, and bZIP23). Moreover, this strain improved yield parameters compared to other treatments and also confirmed its leaf surface colonization. Overall, this study indicates that the foliar application of B. megaterium PB50 can induce tolerance to drought stress in rice.
Keywords: Bacillus megaterium PB50; drought stress; induced systemic tolerance; phyllosphere; plant-growth-promoting bacteria.
Conflict of interest statement
The authors declare that there is no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
Application of data integration for rice bacterial strain selection by combining their osmotic stress response and plant growth-promoting traits.Front Microbiol. 2022 Dec 15;13:1058772. doi: 10.3389/fmicb.2022.1058772. eCollection 2022. Front Microbiol. 2022. PMID: 36590400 Free PMC article.
-
Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria.J Basic Microbiol. 2020 Sep;60(9):768-786. doi: 10.1002/jobm.202000011. Epub 2020 Jul 15. J Basic Microbiol. 2020. PMID: 32667057
-
Plant growth-promoting rhizobacterium Bacillus megaterium modulates the expression of antioxidant-related and drought-responsive genes to protect rice (Oryza sativa L.) from drought.Front Microbiol. 2024 Aug 21;15:1430546. doi: 10.3389/fmicb.2024.1430546. eCollection 2024. Front Microbiol. 2024. PMID: 39234545 Free PMC article.
-
Foliar spray of silica improved water stress tolerance in rice (Oryza sativa L.) cultivars.Front Plant Sci. 2022 Nov 16;13:935090. doi: 10.3389/fpls.2022.935090. eCollection 2022. Front Plant Sci. 2022. PMID: 36466243 Free PMC article.
-
A consortium of rhizobacterial strains and biochemical growth elicitors improve cold and drought stress tolerance in rice (Oryza sativa L.).Plant Biol (Stuttg). 2016 May;18(3):471-83. doi: 10.1111/plb.12427. Epub 2016 Jan 26. Plant Biol (Stuttg). 2016. PMID: 26681628
Cited by
-
Pan-metagenome reveals the abiotic stress resistome of cigar tobacco phyllosphere microbiome.Front Plant Sci. 2023 Dec 21;14:1248476. doi: 10.3389/fpls.2023.1248476. eCollection 2023. Front Plant Sci. 2023. PMID: 38179476 Free PMC article.
-
Management of Rhizosphere Microbiota and Plant Production under Drought Stress: A Comprehensive Review.Plants (Basel). 2022 Sep 19;11(18):2437. doi: 10.3390/plants11182437. Plants (Basel). 2022. PMID: 36145836 Free PMC article. Review.
-
Plant-Growth-Promoting Bacteria.Plants (Basel). 2024 May 11;13(10):1323. doi: 10.3390/plants13101323. Plants (Basel). 2024. PMID: 38794394 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of Heat Shock Protein 70 (HSP70) Gene Family in Pumpkin (Cucurbita moschata) Rootstock under Drought Stress Suggested the Potential Role of these Chaperones in Stress Tolerance.Int J Mol Sci. 2022 Feb 8;23(3):1918. doi: 10.3390/ijms23031918. Int J Mol Sci. 2022. PMID: 35163839 Free PMC article.
-
Application of data integration for rice bacterial strain selection by combining their osmotic stress response and plant growth-promoting traits.Front Microbiol. 2022 Dec 15;13:1058772. doi: 10.3389/fmicb.2022.1058772. eCollection 2022. Front Microbiol. 2022. PMID: 36590400 Free PMC article.
References
-
- De Oliveira A.C., Pegoraro C., Viana V.E., editors. The Future of Rice Demand: Quality Beyond Productivity. Springer; Cham, Switzerland: 2020. - DOI
-
- Bouman B.A.M., Tuong T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001;49:11–30. doi: 10.1016/S0378-3774(00)00128-1. - DOI
-
- O’Neill B.C., MacKellar F.L., Lutz W. Population and Climate Change. Cambridge University Press; Cambridge, UK: 2005.
-
- Fitton N., Alexander P., Arnell N., Bajzelj B., Calvin K., Doelman J., Gerber J.S., Havlik P., Hasegawa T., Herrero M., et al. The vulnerabilities of agricultural land and food production to future water scarcity. Global Environ. Chang. 2019;58:101944. doi: 10.1016/j.gloenvcha.2019.101944. - DOI
-
- Lipiec J., Doussan C., Nosalewicz A., Kondracka K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013;27:463–477. doi: 10.2478/intag-2013-0017. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

