Heme Oxygenase 1: A Defensive Mediator in Kidney Diseases
- PMID: 33670516
- PMCID: PMC7923026
- DOI: 10.3390/ijms22042009
Heme Oxygenase 1: A Defensive Mediator in Kidney Diseases
Abstract
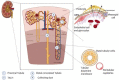
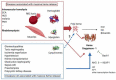
The incidence of kidney disease is rising, constituting a significant burden on the healthcare system and making identification of new therapeutic targets increasingly urgent. The heme oxygenase (HO) system performs an important function in the regulation of oxidative stress and inflammation and, via these mechanisms, is thought to play a role in the prevention of non-specific injuries following acute renal failure or resulting from chronic kidney disease. The expression of HO-1 is strongly inducible by a wide range of stimuli in the kidney, consequent to the kidney's filtration role which means HO-1 is exposed to a wide range of endogenous and exogenous molecules, and it has been shown to be protective in a variety of nephropathological animal models. Interestingly, the positive effect of HO-1 occurs in both hemolysis- and rhabdomyolysis-dominated diseases, where the kidney is extensively exposed to heme (a major HO-1 inducer), as well as in non-heme-dependent diseases such as hypertension, diabetic nephropathy or progression to end-stage renal disease. This highlights the complexity of HO-1's functions, which is also illustrated by the fact that, despite the abundance of preclinical data, no drug targeting HO-1 has so far been translated into clinical use. The objective of this review is to assess current knowledge relating HO-1's role in the kidney and its potential interest as a nephroprotection agent. The potential therapeutic openings will be presented, in particular through the identification of clinical trials targeting this enzyme or its products.
Keywords: heme; heme-oxygenase-1; hemolysis; ischemia reperfusion; kidney; rhabdomyolysis; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Foreman K.J., Marquez N., Dolgert A., Fukutaki K., Fullman N., McGaughey M., Pletcher M.A., Smith A.E., Tang K., Yuan C.-W., et al. Forecasting Life Expectancy, Years of Life Lost, and All-Cause and Cause-Specific Mortality for 250 Causes of Death: Reference and Alternative Scenarios for 2016–40 for 195 Countries and Territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. - DOI - PMC - PubMed
-
- Luyckx V.A., Al-Aly Z., Bello A.K., Bellorin-Font E., Carlini R.G., Fabian J., Garcia-Garcia G., Iyengar A., Sekkarie M., van Biesen W., et al. Sustainable Development Goals Relevant to Kidney Health: An Update on Progress. Nat. Rev. Nephrol. 2020 doi: 10.1038/s41581-020-00363-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

