BMP Receptor Inhibition Enhances Tissue Repair in Endoglin Heterozygous Mice
- PMID: 33670533
- PMCID: PMC7922601
- DOI: 10.3390/ijms22042010
BMP Receptor Inhibition Enhances Tissue Repair in Endoglin Heterozygous Mice
Abstract
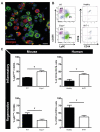
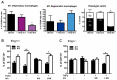
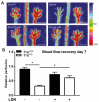
Hereditary hemorrhagic telangiectasia type 1 (HHT1) is a severe vascular disorder caused by mutations in the TGFβ/BMP co-receptor endoglin. Endoglin haploinsufficiency results in vascular malformations and impaired neoangiogenesis. Furthermore, HHT1 patients display an impaired immune response. To date it is not fully understood how endoglin haploinsufficient immune cells contribute to HHT1 pathology. Therefore, we investigated the immune response during tissue repair in Eng+/- mice, a model for HHT1. Eng+/- mice exhibited prolonged infiltration of macrophages after experimentally induced myocardial infarction. Moreover, there was an increased number of inflammatory M1-like macrophages (Ly6Chigh/CD206-) at the expense of reparative M2-like macrophages (Ly6Clow/CD206+). Interestingly, HHT1 patients also showed an increased number of inflammatory macrophages. In vitro analysis revealed that TGFβ-induced differentiation of Eng+/- monocytes into M2-like macrophages was blunted. Inhibiting BMP signaling by treating monocytes with LDN-193189 normalized their differentiation. Finally, LDN treatment improved heart function after MI and enhanced vascularization in both wild type and Eng+/- mice. The beneficial effect of LDN was also observed in the hind limb ischemia model. While blood flow recovery was hampered in vehicle-treated animals, LDN treatment improved tissue perfusion recovery in Eng+/- mice. In conclusion, BMPR kinase inhibition restored HHT1 macrophage imbalance in vitro and improved tissue repair after ischemic injury in Eng+/- mice.
Keywords: endoglin; hind limb ischemia; myocardial infarction; neovascularization; tissue repair; transforming growth factor-β.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pérez-Gómez E., Jerkic M., Prieto M., Del Castillo G., Martín-Villar E., Letarte M., Bernabeu C., Pérez-Barriocanal F., Quintanilla M., López-Novoa J.M. Impaired wound repair in adult endoglin heterozygous mice associated with lower NO bioavailability. J. Investig. Dermatol. 2014;134:247–255. doi: 10.1038/jid.2013.263. - DOI - PubMed
-
- Peter M.R., Jerkic M., Sotov V., Douda D.N., Ardelean D.S., Ghamami N., Lakschevitz F., Khan M.A., Robertson S.J., Glogauer M., et al. Impaired resolution of inflammation in the endoglin heterozygous mouse model of chronic colitis. Mediat. Inflamm. 2014;2014:1–13. doi: 10.1155/2014/767185. - DOI - PMC - PubMed
-
- van Laake L.W., van den Driesche S., Post S., Feijen A., Jansen M.A., Driessens M.H., Mager J.J., Snijder R.J., Westermann C.J.J., Doevendans P.A., et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation. 2006;114:2288–2297. doi: 10.1161/CIRCULATIONAHA.106.639161. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

