The Radiation-Induced Regenerative Response of Adult Tissue-Specific Stem Cells: Models and Signaling Pathways
- PMID: 33670536
- PMCID: PMC7921940
- DOI: 10.3390/cancers13040855
The Radiation-Induced Regenerative Response of Adult Tissue-Specific Stem Cells: Models and Signaling Pathways
Abstract
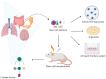
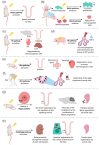
Radiotherapy is involved in the treatment of many cancers, but damage induced to the surrounding normal tissue is often inevitable. Evidence suggests that the maintenance of homeostasis and regeneration of the normal tissue is driven by specific adult tissue stem/progenitor cells. These tasks involve the input from several signaling pathways. Irradiation also targets these stem/progenitor cells, triggering a cellular response aimed at achieving tissue regeneration. Here we discuss the currently used in vitro and in vivo models and the involved specific tissue stem/progenitor cell signaling pathways to study the response to irradiation. The combination of the use of complex in vitro models that offer high in vivo resemblance and lineage tracing models, which address organ complexity constitute potential tools for the study of the stem/progenitor cellular response post-irradiation. The Notch, Wnt, Hippo, Hedgehog, and autophagy signaling pathways have been found as crucial for driving stem/progenitor radiation-induced tissue regeneration. We review how these signaling pathways drive the response of solid tissue-specific stem/progenitor cells to radiotherapy and the used models to address this.
Keywords: radiotherapy; regeneration; signaling pathways; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Combining transfusion of stem/progenitor cells into the peripheral circulation with localized transplantation in situ at the site of tissue/organ damage: a possible strategy to optimize the efficacy of stem cell transplantation therapy.Med Hypotheses. 2005;65(3):494-7. doi: 10.1016/j.mehy.2005.04.003. Med Hypotheses. 2005. PMID: 15982831
-
Radiation-Induced Tissue Regeneration: Pathways, Mechanisms, and Therapeutic Potential.Hematol Oncol Clin North Am. 2025 Apr;39(2):431-452. doi: 10.1016/j.hoc.2024.12.003. Epub 2025 Jan 17. Hematol Oncol Clin North Am. 2025. PMID: 39827040 Review.
-
The periodontal stem/progenitor cell inflammatory-regenerative cross talk: A new perspective.J Periodontal Res. 2019 Apr;54(2):81-94. doi: 10.1111/jre.12616. Epub 2018 Oct 8. J Periodontal Res. 2019. PMID: 30295324 Review.
-
The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration.Endocrinology. 2012 Jul;153(7):3224-35. doi: 10.1210/en.2012-1152. Epub 2012 Apr 19. Endocrinology. 2012. PMID: 22518061
-
Targeting stem cell signaling pathways for drug discovery: advances in the Notch and Wnt pathways.Sci China Life Sci. 2014 Jun;57(6):575-80. doi: 10.1007/s11427-014-4665-7. Epub 2014 May 15. Sci China Life Sci. 2014. PMID: 24829106 Review.
Cited by
-
Radiation decreases bronchial epithelial progenitor function as assessed by organoid formation.Respir Res. 2025 Jan 18;26(1):20. doi: 10.1186/s12931-025-03105-z. Respir Res. 2025. PMID: 39827355 Free PMC article.
-
Aberrant ATM signaling and homology-directed DNA repair as a vulnerability of p53-mutant GBM to AZD1390-mediated radiosensitization.Sci Transl Med. 2024 Feb 14;16(734):eadj5962. doi: 10.1126/scitranslmed.adj5962. Epub 2024 Feb 14. Sci Transl Med. 2024. PMID: 38354228 Free PMC article.
-
Intestinal Epithelial Regeneration in Response to Ionizing Irradiation.J Vis Exp. 2022 Jul 27;(185):10.3791/64028. doi: 10.3791/64028. J Vis Exp. 2022. PMID: 35969101 Free PMC article.
-
Renin-Angiotensin Inhibitor, Captopril, Attenuates Growth of Patient-Derived Colorectal Liver Metastasis Organoids.Int J Mol Sci. 2024 Mar 14;25(6):3282. doi: 10.3390/ijms25063282. Int J Mol Sci. 2024. PMID: 38542253 Free PMC article.
-
Colonic crypt stem cell functions are controlled by tight junction protein claudin-7 through Notch/Hippo signaling.Ann N Y Acad Sci. 2024 May;1535(1):92-108. doi: 10.1111/nyas.15137. Epub 2024 Apr 10. Ann N Y Acad Sci. 2024. PMID: 38598500 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

