Weakly Acidic Bile Is a Risk Factor for Hypopharyngeal Carcinogenesis Evidenced by DNA Damage, Antiapoptotic Function, and Premalignant Dysplastic Lesions In Vivo
- PMID: 33670587
- PMCID: PMC7923205
- DOI: 10.3390/cancers13040852
Weakly Acidic Bile Is a Risk Factor for Hypopharyngeal Carcinogenesis Evidenced by DNA Damage, Antiapoptotic Function, and Premalignant Dysplastic Lesions In Vivo
Abstract
Background: There is recent in vivo discovery documenting the carcinogenic effect of bile at strongly acidic pH 3.0 in hypopharynx, while in vitro data demonstrate that weakly acidic bile (pH 5.5) has a similar oncogenic effect. Because esophageal refluxate often occurs at pH > 4.0, here we aim to determine whether weakly acidic bile is also carcinogenic in vivo.
Methods: Using 32 wild-type mice C57B16J, we performed topical application of conjugated primary bile acids with or without unconjugated secondary bile acid, deoxycholic acid (DCA), at pH 5.5 and controls, to hypopharyngeal mucosa (HM) twice per day, for 15 weeks.
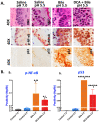
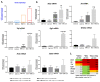
Results: Chronic exposure of HM to weakly acidic bile, promotes premalignant lesions with microinvasion, preceded by significant DNA/RNA oxidative damage, γH2AX (double strand breaks), NF-κB and p53 expression, overexpression of Bcl-2, and elevated Tnf and Il6 mRNAs, compared to controls. Weakly acidic bile, without DCA, upregulates the "oncomirs", miR-21 and miR-155. The presence of DCA promotes Egfr, Wnt5a, and Rela overexpression, and a significant downregulation of "tumor suppressor" miR-451a.
Conclusion: Weakly acidic pH increases the risk of bile-related hypopharyngeal neoplasia. The oncogenic properties of biliary esophageal reflux on the epithelium of the upper aerodigestive tract may not be fully modified when antacid therapy is applied. We believe that due to bile content, alternative therapeutic strategies using specific inhibitors of relevant molecular pathways or receptors may be considered in patients with refractory GERD.
Keywords: DNA damage; NF-κB; bile; head and neck cancer; hypopharyngeal cancer; in vivo; laryngopharyngeal reflux; weakly acidic reflux.
Conflict of interest statement
The authors whose names are listed in this article certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures



References
-
- Langevin S.M., Michaud D.S., Marsit C.J., Nelson H.H., Birnbaum A.E., Eliot M., Christensen B.C., McClean M.D., Kelsey K.T. Gastric reflux is an independent risk factor for laryngopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2013;22:1061–1068. doi: 10.1158/1055-9965.EPI-13-0183. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

