Loganin Alleviates Gout Inflammation by Suppressing NLRP3 Inflammasome Activation and Mitochondrial Damage
- PMID: 33670601
- PMCID: PMC7923023
- DOI: 10.3390/molecules26041071
Loganin Alleviates Gout Inflammation by Suppressing NLRP3 Inflammasome Activation and Mitochondrial Damage
Abstract
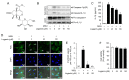
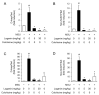
Gout is a type of inflammatory arthritis caused by the deposition of monosodium uric acid (MSU) crystals in tissues. The etiology of gout is directly linked to the NLRP3 inflammasome, since MSU crystals are NLRP3 inflammasome activators. Therefore, we decided to search for a small-molecule inhibitor of the NLRP3 inflammasome for the prevention of gout inflammation. We found that loganin suppressed MSU crystals-induced caspase-1 (p20) and interleukin (IL)-1β production and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) specks formation in mouse primary macrophages, showing its ability to inhibit the NLRP3 inflammasome. In an air pouch inflammation model, oral administration of loganin to mice prevented MSU crystals-induced production of mature IL-1β and IL-18 in air pouch exudates, resulting in decreased neutrophil recruitment. Furthermore, oral administration of loganin suppressed MSU crystals-induced gout inflammation in a mouse foot gout model, which was accompanied by the inhibition of the NLRP3 inflammasome. Loganin blocked de novo synthesis of mitochondrial DNA in air pouches and foot tissues injected with MSU crystals. Consistently, loganin prevented MSU crystals-induced mitochondrial damage in macrophages, as it increased mitochondrial membrane potential and decreased the amount of mitochondrial reactive oxygen species. These data demonstrate that loganin suppresses NLRP3 inflammasome activation by inhibiting mitochondrial stress. These results suggest a novel pharmacological strategy to prevent gout inflammation by blocking NLRP3 inflammasome activation and mitochondrial dysfunction.
Keywords: cytokine; inflammation; innate immunity; mitochondria; pharmacological inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial DNA Synthesis.Molecules. 2019 Jun 6;24(11):2138. doi: 10.3390/molecules24112138. Molecules. 2019. PMID: 31174271 Free PMC article.
-
Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation.Rheumatology (Oxford). 2018 Apr 1;57(4):727-736. doi: 10.1093/rheumatology/kex499. Rheumatology (Oxford). 2018. PMID: 29340626
-
Management of Gout-associated MSU crystals-induced NLRP3 inflammasome activation by procyanidin B2: targeting IL-1β and Cathepsin B in macrophages.Inflammopharmacology. 2020 Dec;28(6):1481-1493. doi: 10.1007/s10787-020-00758-8. Epub 2020 Oct 1. Inflammopharmacology. 2020. PMID: 33006110
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Beneficial Properties of Phytochemicals on NLRP3 Inflammasome-Mediated Gout and Complication.J Agric Food Chem. 2018 Jan 31;66(4):765-772. doi: 10.1021/acs.jafc.7b05113. Epub 2018 Jan 17. J Agric Food Chem. 2018. PMID: 29293001 Review.
Cited by
-
A novel NLRP3 inhibitor as a therapeutic agent against monosodium urate-induced gout.Front Immunol. 2024 Feb 2;14:1307739. doi: 10.3389/fimmu.2023.1307739. eCollection 2023. Front Immunol. 2024. PMID: 38371945 Free PMC article.
-
Carvacrol Alleviates Hyperuricemia-Induced Oxidative Stress and Inflammation by Modulating the NLRP3/NF-κB Pathwayt.Drug Des Devel Ther. 2022 Apr 22;16:1159-1170. doi: 10.2147/DDDT.S343978. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35496367 Free PMC article.
-
Targeting Neutrophil Extracellular Traps in Gouty Arthritis: Insights into Pathogenesis and Therapeutic Potential.J Inflamm Res. 2024 Mar 19;17:1735-1763. doi: 10.2147/JIR.S460333. eCollection 2024. J Inflamm Res. 2024. PMID: 38523684 Free PMC article. Review.
-
Role of NLRP3 in the pathogenesis and treatment of gout arthritis.Front Immunol. 2023 Mar 27;14:1137822. doi: 10.3389/fimmu.2023.1137822. eCollection 2023. Front Immunol. 2023. PMID: 37051231 Free PMC article.
-
RIG-I Deficiency Promotes Obesity-Induced Insulin Resistance.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1178. doi: 10.3390/ph14111178. Pharmaceuticals (Basel). 2021. PMID: 34832960 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

