Polymeric Materials with Antibacterial Activity: A Review
- PMID: 33670638
- PMCID: PMC7922637
- DOI: 10.3390/polym13040613
Polymeric Materials with Antibacterial Activity: A Review
Abstract
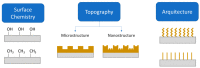
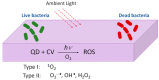
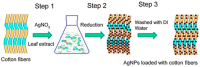
Infections caused by bacteria are one of the main causes of mortality in hospitals all over the world. Bacteria can grow on many different surfaces and when this occurs, and bacteria colonize a surface, biofilms are formed. In this context, one of the main concerns is biofilm formation on medical devices such as urinary catheters, cardiac valves, pacemakers or prothesis. The development of bacteria also occurs on materials used for food packaging, wearable electronics or the textile industry. In all these applications polymeric materials are usually present. Research and development of polymer-based antibacterial materials is crucial to avoid the proliferation of bacteria. In this paper, we present a review about polymeric materials with antibacterial materials. The main strategies to produce materials with antibacterial properties are presented, for instance, the incorporation of inorganic particles, micro or nanostructuration of the surfaces and antifouling strategies are considered. The antibacterial mechanism exerted in each case is discussed. Methods of materials preparation are examined, presenting the main advantages or disadvantages of each one based on their potential uses. Finally, a review of the main characterization techniques and methods used to study polymer based antibacterial materials is carried out, including the use of single force cell spectroscopy, contact angle measurements and surface roughness to evaluate the role of the physicochemical properties and the micro or nanostructure in antibacterial behavior of the materials.
Keywords: antibacterial; biomedicine; food science; nanoparticles; polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Prevention of Bacterial Colonization on Catheters by a One-Step Coating Process Involving an Antibiofouling Polymer in Water.ACS Appl Mater Interfaces. 2017 Jun 14;9(23):19736-19745. doi: 10.1021/acsami.7b06899. Epub 2017 Jun 1. ACS Appl Mater Interfaces. 2017. PMID: 28569502
-
Physical methods for controlling bacterial colonization on polymer surfaces.Biotechnol Adv. 2020 Nov 1;43:107586. doi: 10.1016/j.biotechadv.2020.107586. Epub 2020 Jul 12. Biotechnol Adv. 2020. PMID: 32663616 Review.
-
Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles.Mater Sci Eng C Mater Biol Appl. 2020 Mar;108:110319. doi: 10.1016/j.msec.2019.110319. Epub 2019 Oct 23. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31923962 Review.
-
Antibiofilm Effect of Poly(Vinyl Alcohol-co-Ethylene) Halamine Film against Listeria innocua and Escherichia coli O157:H7.Appl Environ Microbiol. 2017 Sep 15;83(19):e00975-17. doi: 10.1128/AEM.00975-17. Print 2017 Oct 1. Appl Environ Microbiol. 2017. PMID: 28802271 Free PMC article.
-
Physico-chemistry of bacterial transmission versus adhesion.Adv Colloid Interface Sci. 2017 Dec;250:15-24. doi: 10.1016/j.cis.2017.11.002. Epub 2017 Nov 5. Adv Colloid Interface Sci. 2017. PMID: 29129313 Review.
Cited by
-
Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis.Polymers (Basel). 2024 Sep 5;16(17):2526. doi: 10.3390/polym16172526. Polymers (Basel). 2024. PMID: 39274158 Free PMC article. Review.
-
Green Synthesis of Silver Nanoparticles using Cirsium congestum Extract Modified by Chitosan/Alginate: Bactericidal Activity against Pathogenic Bacteria and Cytotoxicity Analysis in Normal Cell Line.Curr Pharm Des. 2024;30(20):1610-1623. doi: 10.2174/0113816128304460240408085736. Curr Pharm Des. 2024. PMID: 38661036
-
Comparative Experimental Investigation of Biodegradable Antimicrobial Polymer-Based Composite Produced by 3D Printing Technology Enriched with Metallic Particles.Int J Mol Sci. 2022 Sep 23;23(19):11235. doi: 10.3390/ijms231911235. Int J Mol Sci. 2022. PMID: 36232537 Free PMC article.
-
Biosafety chemistry and biosafety materials: A new perspective to solve biosafety problems.Biosaf Health. 2022 Feb;4(1):15-22. doi: 10.1016/j.bsheal.2022.01.001. Epub 2022 Jan 4. Biosaf Health. 2022. PMID: 35013725 Free PMC article. Review.
-
Intrinsically Disordered Synthetic Polymers in Biomedical Applications.Polymers (Basel). 2023 May 22;15(10):2406. doi: 10.3390/polym15102406. Polymers (Basel). 2023. PMID: 37242981 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

