Bioconjugation of a PNA Probe to Zinc Oxide Nanowires for Label-Free Sensing
- PMID: 33670746
- PMCID: PMC7923052
- DOI: 10.3390/nano11020523
Bioconjugation of a PNA Probe to Zinc Oxide Nanowires for Label-Free Sensing
Abstract
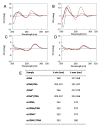
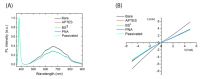
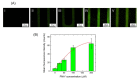
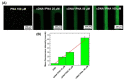
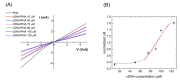
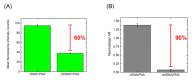
Zinc oxide nanowires (ZnONWs) are largely used in biosensing applications due to their large specific surface area, photoluminescence emission and electron mobility. In this work, the surfaces of ZnONWs are modified by covalent bioconjugation of a peptidic nucleic acid (PNA) probe whose sequence is properly chosen to recognize a complementary DNA (cDNA) strand corresponding to a tract of the CD5 mRNA, the main prognostic marker of chronic lymphatic leukemia. The interaction between PNA and cDNA is preliminarily investigated in solution by circular dichroism, CD melting, and polyacrylamide gel electrophoresis. After the immobilization of the PNA probe on the ZnONW surface, we demonstrate the ability of the PNA-functionalized ZnONW platform to detect cDNA in the μM range of concentration by electrical, label-free measurements. The specificity of the sensor is also verified against a non-complementary DNA sequence. These preliminary results highlight the potential application of PNA-bioconjugated ZnONWs to label-free biosensing of tumor markers.
Keywords: DNA; PNA probe; ZnO nanowire; label-free biosensing; surface functionalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

