Exosomal Cargo May Hold the Key to Improving Reproductive Outcomes in Dairy Cows
- PMID: 33670752
- PMCID: PMC7922264
- DOI: 10.3390/ijms22042024
Exosomal Cargo May Hold the Key to Improving Reproductive Outcomes in Dairy Cows
Abstract
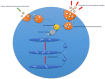
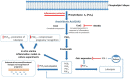
The reproductive status of dairy cows remains a challenge for dairy farmers worldwide, with impaired fertility linked to a significant reduction in herd profitability, due in part to impaired immunity, increased metabolic pressure, and longer postpartum anestrous interval (PPAI). Exosomes are nanovesicles released from a variety of cell types and end up in circulation, and carry proteins, bioactive peptides, lipids, and nucleic acids specific to the place of origin. As such, their role in health and disease has been investigated in humans and animals. This review discusses research into exosomes in the context of reproduction in dairy herds and introduces recent advances in mass-spectrometry (MS) based proteomics that have a potential to advance quantitative profiling of exosomal protein cargo in a search for early biomarkers of cattle fertility.
Keywords: SWATH; dairy cow; exosome; fertility; mass-spectrometry; proteomics; reproduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mitchell M.D., Crookenden M.A., Vaswani K., Roche J.R., Peiris H.N. The frontiers of biomedical science and its application to animal science in addressing the major challenges facing Australasian dairy farming. Anim. Prod. Sci. 2020;60 doi: 10.1071/AN18579. - DOI
-
- Koh Y.Q., Peiris H.N., Vaswani K., Almughlliq F.B., Meier S., Burke C.R., Roche J.R., Reed C.B., Arachchige B.J., Reed S., et al. Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. J. Dairy Sci. 2018;101:6462–6473. doi: 10.3168/jds.2017-14190. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

