The Role of Butyrylcholinesterase and Iron in the Regulation of Cholinergic Network and Cognitive Dysfunction in Alzheimer's Disease Pathogenesis
- PMID: 33670778
- PMCID: PMC7922581
- DOI: 10.3390/ijms22042033
The Role of Butyrylcholinesterase and Iron in the Regulation of Cholinergic Network and Cognitive Dysfunction in Alzheimer's Disease Pathogenesis
Abstract
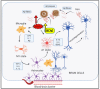
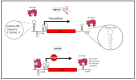
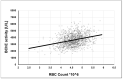
Alzheimer's disease (AD), the most common form of dementia in elderly individuals, is marked by progressive neuron loss. Despite more than 100 years of research on AD, there is still no treatment to cure or prevent the disease. High levels of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) in the brain are neuropathological hallmarks of AD. However, based on postmortem analyses, up to 44% of individuals have been shown to have high Aβ deposits with no clinical signs, due to having a "cognitive reserve". The biochemical mechanism explaining the prevention of cognitive impairment in the presence of Aβ plaques is still unknown. It seems that in addition to protein aggregation, neuroinflammatory changes associated with aging are present in AD brains that are correlated with a higher level of brain iron and oxidative stress. It has been shown that iron accumulates around amyloid plaques in AD mouse models and postmortem brain tissues of AD patients. Iron is required for essential brain functions, including oxidative metabolism, myelination, and neurotransmitter synthesis. However, an imbalance in brain iron homeostasis caused by aging underlies many neurodegenerative diseases. It has been proposed that high iron levels trigger an avalanche of events that push the progress of the disease, accelerating cognitive decline. Patients with increased amyloid plaques and iron are highly likely to develop dementia. Our observations indicate that the butyrylcholinesterase (BChE) level seems to be iron-dependent, and reports show that BChE produced by reactive astrocytes can make cognitive functions worse by accelerating the decay of acetylcholine in aging brains. Why, even when there is a genetic risk, do symptoms of the disease appear after many years? Here, we discuss the relationship between genetic factors, age-dependent iron tissue accumulation, and inflammation, focusing on AD.
Keywords: Alzheimer’s disease; BChE; BuChE; IRE; amyloid; butyrylcholinesterase; iron; neuroinflammation; pseudocholinesterase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Quantification of Butyrylcholinesterase Activity as a Sensitive and Specific Biomarker of Alzheimer's Disease.J Alzheimers Dis. 2017;58(2):491-505. doi: 10.3233/JAD-170164. J Alzheimers Dis. 2017. PMID: 28453492 Free PMC article.
-
Interaction of exogenous acetylcholinesterase and butyrylcholinesterase with amyloid-β plaques in human brain tissue.Chem Biol Interact. 2024 May 25;395:111012. doi: 10.1016/j.cbi.2024.111012. Epub 2024 Apr 20. Chem Biol Interact. 2024. PMID: 38648920
-
Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer's disease.Brain Res. 2017 Sep 15;1671:102-110. doi: 10.1016/j.brainres.2017.07.009. Epub 2017 Jul 17. Brain Res. 2017. PMID: 28729192
-
Butyrylcholinesterase as a Diagnostic and Therapeutic Target for Alzheimer's Disease.Curr Alzheimer Res. 2016;13(10):1173-7. doi: 10.2174/1567205013666160404120542. Curr Alzheimer Res. 2016. PMID: 27040140 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Repurposing Duloxetine as a Potent Butyrylcholinesterase Inhibitor: Potential Cholinergic Enhancing Benefits for Elderly Individuals with Depression and Cognitive Impairment.ACS Omega. 2024 Aug 21;9(35):37299-37309. doi: 10.1021/acsomega.4c05089. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246500 Free PMC article.
-
Serotonin 5-HT6 Receptor Ligands and Butyrylcholinesterase Inhibitors Displaying Antioxidant Activity-Design, Synthesis and Biological Evaluation of Multifunctional Agents against Alzheimer's Disease.Int J Mol Sci. 2022 Aug 21;23(16):9443. doi: 10.3390/ijms23169443. Int J Mol Sci. 2022. PMID: 36012707 Free PMC article.
-
Exploring the Benzazoles Derivatives as Pharmacophores for AChE, BACE1, and as Anti-Aβ Aggregation to Find Multitarget Compounds against Alzheimer's Disease.Molecules. 2024 Oct 9;29(19):4780. doi: 10.3390/molecules29194780. Molecules. 2024. PMID: 39407708 Free PMC article. Review.
-
Serum butyrylcholinesterase activity as a predictor of severity and mortality in COVID-19 patients.Sci Rep. 2025 Jul 2;15(1):23437. doi: 10.1038/s41598-025-07017-2. Sci Rep. 2025. PMID: 40604014 Free PMC article.
-
Design, synthesis and evaluation of quinoline-O-carbamate derivatives as multifunctional agents for the treatment of Alzheimer's disease.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2169682. doi: 10.1080/14756366.2023.2169682. J Enzyme Inhib Med Chem. 2023. PMID: 36688444 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

