Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues
- PMID: 33670791
- PMCID: PMC7922983
- DOI: 10.3390/molecules26041081
Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues
Abstract
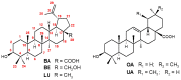
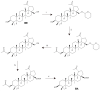
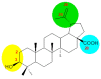
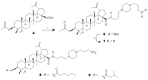
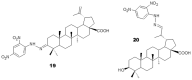
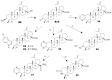
Betulinic acid (BA, 3β-hydroxy-lup-20(29)-en-28-oic acid) is a pentacyclic triterpene acid present predominantly in Betula ssp. (Betulaceae) and is also widely spread in many species belonging to different plant families. BA presents a wide spectrum of remarkable pharmacological properties, such as cytotoxic, anti-HIV, anti-inflammatory, antidiabetic and antimicrobial activities, including antiprotozoal effects. The present review first describes the sources of BA and discusses the chemical strategies to produce this molecule starting from betulin, its natural precursor. Next, the antiprotozoal properties of BA are briefly discussed and the chemical strategies for the synthesis of analogues displaying antiplasmodial, antileishmanial and antitrypanosomal activities are systematically presented. The antiplasmodial activity described for BA was moderate, nevertheless, some C-3 position acylated analogues showed an improvement of this activity and the hybrid models-with artesunic acid-showed the most interesting properties. Some analogues also presented more intense antileishmanial activities compared with BA, and, in addition to these, heterocycles fused to C-2/C-3 positions and amide derivatives were the most promising analogues. Regarding the antitrypanosomal activity, some interesting antitrypanosomal derivatives were prepared by amide formation at the C-28 carboxylic group of the lupane skeleton. Considering that BA can be produced either by isolation of different plant extracts or by chemical transformation of betulin, easily obtained from Betula ssp., it could be said that BA is a molecule of great interest as a starting material for the synthesis of novel antiprotozoal agents.
Keywords: antileishmanial activity; antiplasmodial activity; antiprotozoal activities; antitrypanosomal activity; betulinic acid; triterpene acid derivatives.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
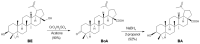

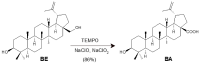





Similar articles
-
Antiprotozoal activity of betulinic acid derivatives.Phytomedicine. 2010 Apr;17(5):379-82. doi: 10.1016/j.phymed.2009.08.002. Epub 2009 Sep 11. Phytomedicine. 2010. PMID: 19748254
-
Anti-leishmanial activity of betulin derivatives.J Antibiot (Tokyo). 2010 Mar;63(3):123-6. doi: 10.1038/ja.2010.2. Epub 2010 Feb 5. J Antibiot (Tokyo). 2010. PMID: 20139867
-
Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine.PLoS One. 2014 Mar 18;9(3):e89939. doi: 10.1371/journal.pone.0089939. eCollection 2014. PLoS One. 2014. PMID: 24643019 Free PMC article.
-
Betulinic Acid and its Derivatives as Potential Antitumor Agents.Med Res Rev. 2015 Nov;35(6):1127-55. doi: 10.1002/med.21353. Epub 2015 Jun 2. Med Res Rev. 2015. PMID: 26032847 Review.
-
Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds.Molecules. 2019 Jan 19;24(2):355. doi: 10.3390/molecules24020355. Molecules. 2019. PMID: 30669472 Free PMC article. Review.
Cited by
-
The Anti-Inflammatory Effect of Zhibaidihuang Decoction on Recurrent Oral Ulcer with Sirt1 as the Key Regulatory Target.Evid Based Complement Alternat Med. 2021 May 3;2021:8886699. doi: 10.1155/2021/8886699. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34007301 Free PMC article.
-
Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii.Antibiotics (Basel). 2022 Feb 14;11(2):248. doi: 10.3390/antibiotics11020248. Antibiotics (Basel). 2022. PMID: 35203850 Free PMC article.
-
Methods of Analysis and Identification of Betulin and Its Derivatives.Molecules. 2023 Aug 8;28(16):5946. doi: 10.3390/molecules28165946. Molecules. 2023. PMID: 37630198 Free PMC article.
-
Betulinic Acid for Glioblastoma Treatment: Reality, Challenges and Perspectives.Int J Mol Sci. 2024 Feb 9;25(4):2108. doi: 10.3390/ijms25042108. Int J Mol Sci. 2024. PMID: 38396785 Free PMC article. Review.
-
Biocatalysis in the Chemistry of Lupane Triterpenoids.Molecules. 2021 Apr 14;26(8):2271. doi: 10.3390/molecules26082271. Molecules. 2021. PMID: 33919839 Free PMC article. Review.
References
-
- Robertson A., Soliman G., Owen E.C. Polyterpenoid compounds. Part, I. Betulic acid from Cornus florida L. J. Chem. Soc. 1939:1267–1273. doi: 10.1039/jr9390001267. - DOI
-
- Kawaguti R., Kim K.W. The constituents of Zyzyphus vulgaris Lamark var. spinosus Bunge. II. Betulinic acid. Yakugaku Zasshi. 1940;60:595–596. doi: 10.1248/yakushi1881.60.11_595. - DOI
-
- Ralph C.S., White D.E. Betulic acid from Syncarpia laurifolia. J. Chem. Soc. 1949:3433–3434. doi: 10.1039/jr9490003433. - DOI
-
- Retzlaff F. Ueber Herba gratiolae. Arch. Pharm. (Weinh.). 1902;240:561–568. doi: 10.1002/ardp.19022400802. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

