4-Hydroxynonenal Contributes to Fibroblast Senescence in Skin Photoaging Evoked by UV-A Radiation
- PMID: 33670907
- PMCID: PMC7997366
- DOI: 10.3390/antiox10030365
4-Hydroxynonenal Contributes to Fibroblast Senescence in Skin Photoaging Evoked by UV-A Radiation
Abstract
Solar ultraviolet A (UV-A) radiation promotes a huge variety of damages on connective tissues and dermal fibroblasts, including cellular senescence, a major contributor of skin photoaging. The mechanisms of skin photoaging evoked by UV-A partly involve the generation of reactive oxygen species and lipid peroxidation. We previously reported that 4-hydroxynonenal (HNE), a lipid peroxidation-derived aldehyde, forms adducts on elastin in the skins of UV-A irradiated hairless mice, possibly contributing to actinic elastosis. In the present study, we investigated whether and how HNE promotes fibroblast senescence in skin photoaging. Dermal fibroblasts of skins from UV-A-exposed hairless mice exhibited an increased number of γH2AX foci characteristic of cell senescence, together with an accumulation of HNE adducts partly colocalizing with the cytoskeletal protein vimentin. Murine fibroblasts exposed to UV-A radiation (two cycles of 15 J/cm2), or HNE (30 µM, 4 h), exhibited senescence patterns characterized by an increased γH2AX foci expression, an accumulation of acetylated proteins, and a decreased expression of the sirtuin SIRT1. HNE adducts were detected on vimentin in cultured fibroblasts irradiated by UV-A or incubated with HNE. The HNE scavenger carnosine prevented both vimentin modification and fibroblast senescence evoked by HNE in vitro and in the skins of UV-A-exposed mice. Altogether, these data emphasize the role of HNE and lipid peroxidation-derived aldehydes in fibroblast senescence, and confirm the protective effect of carnosine in skin photoaging.
Keywords: 4-hydroxynonenal; UV-A; carnosine; fibroblasts; photoaging; senescence; skin; vimentin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
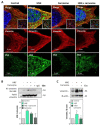
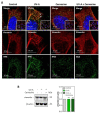
Similar articles
-
Assessing the Role of Carbonyl Adducts, Particularly Malondialdehyde Adducts, in the Development of Dermis Yellowing Occurring during Skin Photoaging.Life (Basel). 2022 Mar 10;12(3):403. doi: 10.3390/life12030403. Life (Basel). 2022. PMID: 35330154 Free PMC article.
-
Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging.Antioxidants (Basel). 2022 Nov 18;11(11):2281. doi: 10.3390/antiox11112281. Antioxidants (Basel). 2022. PMID: 36421467 Free PMC article. Review.
-
Elastin Modification by 4-Hydroxynonenal in Hairless Mice Exposed to UV-A. Role in Photoaging and Actinic Elastosis.J Invest Dermatol. 2015 Jul;135(7):1873-1881. doi: 10.1038/jid.2015.84. Epub 2015 Mar 4. J Invest Dermatol. 2015. PMID: 25739050
-
A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction.Free Radic Biol Med. 2021 Feb 20;164:303-314. doi: 10.1016/j.freeradbiomed.2021.01.002. Epub 2021 Jan 12. Free Radic Biol Med. 2021. PMID: 33450376
-
Solar UV irradiation and dermal photoaging.J Photochem Photobiol B. 2001 Oct;63(1-3):41-51. doi: 10.1016/s1011-1344(01)00201-9. J Photochem Photobiol B. 2001. PMID: 11684450 Review.
Cited by
-
A single dose of Ultraviolet-A induces proteome remodeling and senescence in primary human keratinocytes.Sci Rep. 2021 Dec 2;11(1):23355. doi: 10.1038/s41598-021-02658-5. Sci Rep. 2021. PMID: 34857819 Free PMC article.
-
Reactive Carbonyl Species and Protein Adducts: Identification Strategies, Biological Mechanisms and Molecular Approaches for Their Detoxification.Antioxidants (Basel). 2021 Apr 28;10(5):690. doi: 10.3390/antiox10050690. Antioxidants (Basel). 2021. PMID: 33924749 Free PMC article.
-
Assessing the Role of Carbonyl Adducts, Particularly Malondialdehyde Adducts, in the Development of Dermis Yellowing Occurring during Skin Photoaging.Life (Basel). 2022 Mar 10;12(3):403. doi: 10.3390/life12030403. Life (Basel). 2022. PMID: 35330154 Free PMC article.
-
Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2.Front Pharmacol. 2022 May 12;13:823881. doi: 10.3389/fphar.2022.823881. eCollection 2022. Front Pharmacol. 2022. PMID: 35645796 Free PMC article. Review.
-
Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging.Antioxidants (Basel). 2022 Nov 18;11(11):2281. doi: 10.3390/antiox11112281. Antioxidants (Basel). 2022. PMID: 36421467 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

