An Extracellular Perspective on CNS Maturation: Perineuronal Nets and the Control of Plasticity
- PMID: 33670945
- PMCID: PMC7957817
- DOI: 10.3390/ijms22052434
An Extracellular Perspective on CNS Maturation: Perineuronal Nets and the Control of Plasticity
Abstract
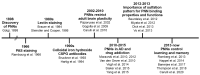
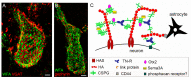
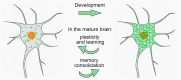
During restricted time windows of postnatal life, called critical periods, neural circuits are highly plastic and are shaped by environmental stimuli. In several mammalian brain areas, from the cerebral cortex to the hippocampus and amygdala, the closure of the critical period is dependent on the formation of perineuronal nets. Perineuronal nets are a condensed form of an extracellular matrix, which surrounds the soma and proximal dendrites of subsets of neurons, enwrapping synaptic terminals. Experimentally disrupting perineuronal nets in adult animals induces the reactivation of critical period plasticity, pointing to a role of the perineuronal net as a molecular brake on plasticity as the critical period closes. Interestingly, in the adult brain, the expression of perineuronal nets is remarkably dynamic, changing its plasticity-associated conditions, including memory processes. In this review, we aimed to address how perineuronal nets contribute to the maturation of brain circuits and the regulation of adult brain plasticity and memory processes in physiological and pathological conditions.
Keywords: Alzheimer’s disease; chondroitin sulfate proteoglycans; critical period; drug addiction; learning; memory; perineuronal net.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

