Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol
- PMID: 33670966
- PMCID: PMC7957601
- DOI: 10.3390/ijms22052438
Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol
Abstract
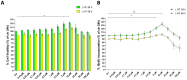
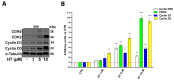
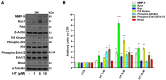
3-hydroxytyrosol (HT) is the main phenolic compound found in olive oil with known antioxidant, anti-inflammatory, and antimicrobial properties in several dermatological conditions, both when taken in the form of olive oil or pure in cosmeceutical formulations. To date, its direct effect on the wound healing process and the molecular mechanisms involved have not yet been elucidated. Thus, in the present study, we aimed to explore its effects in vitro in epidermal keratinocyte cultures focusing on the molecular mechanism implied. HT was able to induce keratinocyte proliferation in the low micromolar range, increasing the expression of cyclin dependent kinases fundamental for cell cycle progression such as CDK2 and CDK6. Furthermore, it increased cell migration through the activation of tissue remodeling factors such as matrix metalloproteinase-9 (MMP-9) protein. Then, we evaluated whether HT also showed antioxidant activity at this concentration range, protecting from H2O2-induced cytotoxicity. The HT prevented the activation of ATM serine/threonine kinase (ATM), Checkpoint kinase 1 (Chk1), Checkpoint kinase 2 (Chk2), and p53, reducing the number of apoptotic cells. Our study highlighted novel pharmacological properties of HT, providing the first evidence of its capability to induce keratinocyte migration and proliferation required for healing processes and re-epithelialization.
Keywords: 3-hydroxytyrosol; cosmeceuticals; oxidative stress; wound healing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury.Pharmaceuticals (Basel). 2020 Aug 29;13(9):224. doi: 10.3390/ph13090224. Pharmaceuticals (Basel). 2020. PMID: 32872510 Free PMC article.
-
Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes.Br J Dermatol. 2008 Nov;159(5):1103-15. doi: 10.1111/j.1365-2133.2008.08789.x. Br J Dermatol. 2008. PMID: 18717678
-
Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: role of Nrf2 activation and HO-1 induction.J Agric Food Chem. 2011 May 11;59(9):4473-82. doi: 10.1021/jf104151d. Epub 2011 Apr 13. J Agric Food Chem. 2011. PMID: 21438539
-
Molecular Action of Hydroxytyrosol in Wound Healing: An In Vitro Evidence-Based Review.Biomolecules. 2020 Sep 30;10(10):1397. doi: 10.3390/biom10101397. Biomolecules. 2020. PMID: 33008084 Free PMC article. Review.
-
Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions.Int J Mol Sci. 2017 Apr 28;18(5):930. doi: 10.3390/ijms18050930. Int J Mol Sci. 2017. PMID: 28452954 Free PMC article. Review.
Cited by
-
Hydroxytyrosol isolation, comparison of synthetic routes and potential biological activities.Food Sci Nutr. 2024 Jul 16;12(10):6899-6912. doi: 10.1002/fsn3.4349. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479663 Free PMC article. Review.
-
Anticancer Activity and Mechanism of Action of Couroupita guianensis Bark Decoction in Gastric Adenocarcinoma Cancer Cell Line.Int J Mol Sci. 2024 Aug 24;25(17):9183. doi: 10.3390/ijms25179183. Int J Mol Sci. 2024. PMID: 39273132 Free PMC article.
-
Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes.Pharmaceuticals (Basel). 2022 Jan 11;15(1):84. doi: 10.3390/ph15010084. Pharmaceuticals (Basel). 2022. PMID: 35056141 Free PMC article.
-
Accelerated wound healing induced by spinach extract in experimental model diabetic rats with streptozotocin.Sci Rep. 2023 Sep 11;13(1):14933. doi: 10.1038/s41598-023-42033-0. Sci Rep. 2023. PMID: 37696865 Free PMC article.
-
Chronic Kidney Disease with Mineral Bone Disorder and Vascular Calcification: An Overview.Life (Basel). 2024 Mar 21;14(3):418. doi: 10.3390/life14030418. Life (Basel). 2024. PMID: 38541742 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

