Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice
- PMID: 33670988
- PMCID: PMC7997284
- DOI: 10.3390/nu13030796
Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice
Abstract
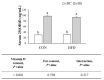
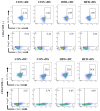
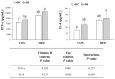
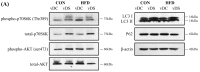
Obesity is associated with an impaired balance of CD4+ T cell subsets. Both vitamin D and obesity have been reported to affect the mTOR pathway. In this study, we investigated the effects of vitamin D on CD4+ T cell subsets and the mTOR pathway. Ten-week-old male C57BL/6 mice were divided into four groups and fed diets with different fat (control or high-fat diets: CON or HFD) and vitamin D contents (vitamin D control or supplemented diets: vDC or vDS) for 12 weeks. T cells purified by negative selection were stimulated with anti-CD3/anti-CD28 mAbs and cultured for 48 h. The percentage of CD4+IL-17+ T cells was higher in the vDS than vDC groups. The CD4+CD25+Foxp3+ T cells percentage was higher in HFD than CON groups. The phospho-p70S6K/total-p70S6K ratio was lower in vDS than vDC, but the phospho-AKT/total-AKT ratio was higher in vDS than vDC groups. Hif1α mRNA levels were lower in vDS than vDC groups. These findings suggest HIF1α plays an important role in vitamin-D-mediated regulation of glucose metabolism in T cells, and dietary vitamin D supplementation may contribute to the maintenance of immune homeostasis by regulating the mTOR pathway in T cells.
Keywords: CD4+ T cell; HIF1α; mTOR pathway; obesity; vitamin D supplementation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

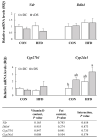
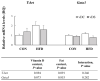
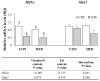
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

