From Submerged Cultures to 3D Cell Culture Models: Evolution of Nasal Epithelial Cells in Asthma Research and Virus Infection
- PMID: 33670992
- PMCID: PMC7997270
- DOI: 10.3390/v13030387
From Submerged Cultures to 3D Cell Culture Models: Evolution of Nasal Epithelial Cells in Asthma Research and Virus Infection
Abstract
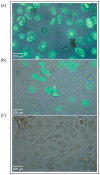
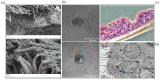
Understanding the response to viral infection in the context of respiratory diseases is of significant importance. Recently, there has been more focus on the role of the nasal epithelium in disease modeling. Here, we provide an overview of different submerged, organotypic 3D and spheroid cell culture models of nasal epithelial cells, which were used to study asthma and allergy with a special focus on virus infection. In detail, this review summarizes the importance, benefits, and disadvantages of patient-derived cell culture models of nasal- and bronchial epithelial cells, including a comparison of these cell culture models and a discussion on why investigators should consider using nasal epithelial cells in their research. Exposure experiments, simple virus transduction analyses as well as genetic studies can be performed in these models, which may provide first insights into the complexity of molecular signatures and may open new doors for drug discovery and biomarker research.
Keywords: 3D cell culture; air–liquid-interface; asthma; bronchial epithelial cells; culture techniques; epithelial cells; nasal epithelial cells; spheroids; submerged; virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. FP receives royalties from Elsevier for the 24th Ed. of the anatomy atlas “Sobotta” and the “Sobotta Textbook of Anatomy”. The German Research Foundation that supported FP (PA738/15-1) had no role in the design or conduct of this research.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

