Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation
- PMID: 33671004
- PMCID: PMC7997353
- DOI: 10.3390/cells10030515
Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation
Abstract
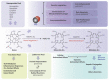
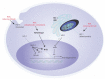
Heme oxygenase catalyzes the rate-limiting step in heme degradation in order to generate biliverdin, carbon monoxide (CO), and iron. The inducible form of the enzyme, heme oxygenase-1 (HO-1), exerts a central role in cellular protection. The substrate, heme, is a potent pro-oxidant that can accelerate inflammatory injury and promote cell death. HO-1 has been implicated as a key mediator of inflammatory cell and tissue injury, as validated in preclinical models of acute lung injury and sepsis. A large body of work has also implicated HO-1 as a cytoprotective molecule against various forms of cell death, including necrosis, apoptosis and newly recognized regulated cell death (RCD) programs such as necroptosis, pyroptosis, and ferroptosis. While the antiapoptotic potential of HO-1 and its reaction product CO in apoptosis regulation has been extensively characterized, relatively fewer studies have explored the regulatory role of HO-1 in other forms of necrotic and inflammatory RCD (i.e., pyroptosis, necroptosis and ferroptosis). HO-1 may provide anti-inflammatory protection in necroptosis or pyroptosis. In contrast, in ferroptosis, HO-1 may play a pro-death role via enhancing iron release. HO-1 has also been implicated in co-regulation of autophagy, a cellular homeostatic program for catabolic recycling of proteins and organelles. While autophagy is primarily associated with cell survival, its occurrence can coincide with RCD programs. This review will summarize the roles of HO-1 and its reaction products in co-regulating RCD and autophagy programs, with its implication for both protective and detrimental tissue responses, with emphasis on how these impact HO-1 as a candidate therapeutic target in disease.
Keywords: apoptosis; autophagy; carbon monoxide; cell death; ferroptosis; heme oxygenase; inflammasome; inflammation; necroptosis; pyroptosis.
Conflict of interest statement
Currently, S.W.R. is a senior scientist role for Proterris, Inc. (Boston). The author declares no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

