Impact of Biological Therapies on the Immune Response after Pneumococcal Vaccination in Patients with Autoimmune Inflammatory Diseases
- PMID: 33671007
- PMCID: PMC7997274
- DOI: 10.3390/vaccines9030203
Impact of Biological Therapies on the Immune Response after Pneumococcal Vaccination in Patients with Autoimmune Inflammatory Diseases
Abstract
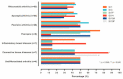
Patients with different autoimmune inflammatory diseases (AIID) on biological therapy are at risk of pneumococcal disease. Adults with inflammatory arthropathies, connective tissue diseases, psoriasis, or inflammatory bowel disease on biological therapy such as anti-TNFα, rituximab, tocilizumab, abatacept, or anakinra were included in this study. Patients completed a protocol combining the pneumococcal vaccines PCV13 and PPV23. Immune response against pneumococcal serotypes 1, 3, 7F, 14, 19A, and 19F were assessed evaluating functional antibodies by an opsonophagocytosis killing assay (OPKA). In this study, 182 patients with AIID completed the sequential vaccination protocol. Patients on etanercept tended to achieve OPKA titers against a larger number of serotypes than the rest of patients on other biological therapies, while adalimumab was associated to a lower number of serotypes with OPKA titers. Rituximab was not associated with a worse response when compared with the rest of biological agents. Not glucocorticoids, nor synthetic disease-modifying antirheumatic drugs, interfered with the immune response. OPKA titers against serotype 3 which is one of the most prevalent, was obtained in 44% of patients, increasing up to 58% in those on etanercept. Hence, almost 50% of patients on biological therapy achieved functional antibodies after the administration of a complete pneumococcal vaccination protocol.
Keywords: OPKA titers; PCV13; PPV23; anti-TNFα; autoimmune inflammatory diseases; biological therapy; invasive pneumococcal disease; rituximab; tocilizumab.
Conflict of interest statement
P.R. declares she has received research grants from Sociedad de Reumatología Comunidad de Madrid (SORCOM)/MSD and Pfizer. J.Y. reports grants from MSD-USA, and personal fees from GSK, MSD and Pfizer, outside the submitted work. S.M.F. declares he has received research grants from Sociedad de Reumatología Comunidad de Madrid (SORCOM)/MSD and Pfizer, and personal fees from Abbvie, Janssen, Novartis, Roche, Sanofi, MSD and Celgene and Pfizer, outside the submitted work. The rest of authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Backhaus E., Berg S., Andersson R., Ockborn G., Malmström P., Dahl M., Nasic S., Trollfors B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016;16:367. doi: 10.1186/s12879-016-1648-2. - DOI - PMC - PubMed
-
- Fernandez-Martinez S., Cortes X., Borrás-Blasco J., Gracia-Pérez A., Casterá M.E. Effectiveness of a systematic vaccination program in patients with autoimmune inflammatory disease treated with anti-TNF alpha drugs. Expert. Opin. Biol. Ther. 2016;16:1317–1322. doi: 10.1080/14712598.2016.1218844. - DOI - PubMed
-
- van Assen S., Agmon-Levin N., Elkayam O., Cervera R., Doran M.F., Dougados M., Emery P., Geborek P., Ioannidis J.P.A., Jayne D.R.W., et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2011;70:414–422. doi: 10.1136/ard.2010.137216. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

