Secreted Signaling Molecules at the Neuromuscular Junction in Physiology and Pathology
- PMID: 33671084
- PMCID: PMC7957818
- DOI: 10.3390/ijms22052455
Secreted Signaling Molecules at the Neuromuscular Junction in Physiology and Pathology
Abstract
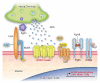
: Signal transduction at the neuromuscular junction (NMJ) is affected in many human diseases, including congenital myasthenic syndromes (CMS), myasthenia gravis, Lambert-Eaton myasthenic syndrome, Isaacs' syndrome, Schwartz-Jampel syndrome, Fukuyama-type congenital muscular dystrophy, amyotrophic lateral sclerosis, and sarcopenia. The NMJ is a prototypic cholinergic synapse between the motor neuron and the skeletal muscle. Synaptogenesis of the NMJ has been extensively studied, which has also been extrapolated to further understand synapse formation in the central nervous system. Studies of genetically engineered mice have disclosed crucial roles of secreted molecules in the development and maintenance of the NMJ. In this review, we focus on the secreted signaling molecules which regulate the clustering of acetylcholine receptors (AChRs) at the NMJ. We first discuss the signaling pathway comprised of neural agrin and its receptors, low-density lipoprotein receptor-related protein 4 (Lrp4) and muscle-specific receptor tyrosine kinase (MuSK). This pathway drives the clustering of acetylcholine receptors (AChRs) to ensure efficient signal transduction at the NMJ. We also discuss three secreted molecules (Rspo2, Fgf18, and connective tissue growth factor (Ctgf)) that we recently identified in the Wnt/β-catenin and fibroblast growth factors (FGF) signaling pathways. The three secreted molecules facilitate the clustering of AChRs by enhancing the agrin-Lrp4-MuSK signaling pathway.
Keywords: FGF signaling; Wnt/β-catenin signaling; acetylcholine receptor; neuromuscular junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- JP17K07094/Grants-in-Aid from the Japan Society for the Promotion of Science
- JP18K06483/Grants-in-Aid from the Japan Society for the Promotion of Science
- JP20H03561/Grants-in-Aid from the Japan Society for the Promotion of Science
- 20FC1036/Ministry of Health, Labour and Welfare
- 20gm1010002/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

