The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter
- PMID: 33671108
- PMCID: PMC7957563
- DOI: 10.3390/ijms22052463
The Novel Benzamide Derivative, VKNG-2, Restores the Efficacy of Chemotherapeutic Drugs in Colon Cancer Cell Lines by Inhibiting the ABCG2 Transporter
Abstract
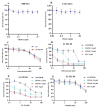
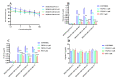
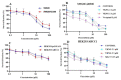
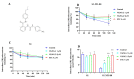
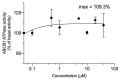
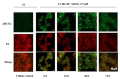
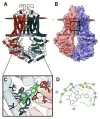
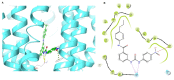
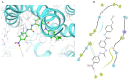
The overexpression of ATP-binding cassette transporter, ABCG2, plays an important role in mediating multidrug resistance (MDR) in certain types of cancer cells. ABCG2-mediated MDR can significantly attenuate or abrogate the efficacy of anticancer drugs by increasing their efflux from cancer cells. In this study, we determined the efficacy of the novel benzamide derivative, VKNG-2, to overcome MDR due to the overexpression of the ABCG2 transporter in the colon cancer cell line, S1-M1-80. In vitro, 5 μM of VKNG-2 reversed the resistance of S1-M1-80 cell line to mitoxantrone (70-fold increase in efficacy) or SN-38 (112-fold increase in efficacy). In contrast, in vitro, 5 μM of VKNG-2 did not significantly alter either the expression of ABCG2, AKT, and PI3K p110β protein or the subcellular localization of the ABCG2 protein compared to colon cancer cells incubated with the vehicle. Molecular docking data indicated that VKNG-2 had a high docking score (-10.2 kcal/mol) for the ABCG2 transporter substrate-drug binding site whereas it had a low affinity on ABCB1 and ABCC1 transporters. Finally, VKNG-2 produced a significant concentration-dependent increase in ATPase activity (EC50 = 2.3 µM). In conclusion, our study suggests that in vitro, VKNG-2 reverses the resistance of S1-M1-80, a cancer cell line resistant to mitoxantrone and SN-38, by inhibiting the efflux function of the ABCG2 transporter.
Keywords: ABCG2; ATP binding cassette; MDR; benzamide; reversal effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Spleen Tyrosine Kinase Inhibitor, Entospletinib (GS-9973) Restores Chemosensitivity in Lung Cancer Cells by Modulating ABCG2-mediated Multidrug Resistance.Int J Biol Sci. 2021 Jun 22;17(10):2652-2665. doi: 10.7150/ijbs.61229. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326700 Free PMC article.
-
Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters.Cancer Lett. 2018 May 1;421:186-198. doi: 10.1016/j.canlet.2018.01.021. Epub 2018 Jan 11. Cancer Lett. 2018. PMID: 29331420
-
VKNG-1 Antagonizes ABCG2-Mediated Multidrug Resistance via p-AKT and Bcl-2 Pathway in Colon Cancer: In Vitro and In Vivo Study.Cancers (Basel). 2021 Sep 17;13(18):4675. doi: 10.3390/cancers13184675. Cancers (Basel). 2021. PMID: 34572902 Free PMC article.
-
Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter ABCG2.Curr Med Chem Anticancer Agents. 2004 Jan;4(1):31-42. doi: 10.2174/1568011043482205. Curr Med Chem Anticancer Agents. 2004. PMID: 14754410 Review.
-
Multidrug efflux transporter ABCG2: expression and regulation.Cell Mol Life Sci. 2021 Nov;78(21-22):6887-6939. doi: 10.1007/s00018-021-03901-y. Epub 2021 Sep 29. Cell Mol Life Sci. 2021. PMID: 34586444 Free PMC article. Review.
Cited by
-
The AKT inhibitor, MK-2206, attenuates ABCG2-mediated drug resistance in lung and colon cancer cells.Front Pharmacol. 2023 Jul 13;14:1235285. doi: 10.3389/fphar.2023.1235285. eCollection 2023. Front Pharmacol. 2023. PMID: 37521473 Free PMC article.
-
Phenylspirodrimane with Moderate Reversal Effect of Multidrug Resistance Isolated from the Deep-Sea Fungus Stachybotrys sp. 3A00409.Molecules. 2024 Apr 8;29(7):1685. doi: 10.3390/molecules29071685. Molecules. 2024. PMID: 38611964 Free PMC article.
-
Establishment and Characterization of a Novel Multidrug Resistant Human Ovarian Cancer Cell Line With Heterogenous MRP7 Overexpression.Front Oncol. 2021 Sep 24;11:731260. doi: 10.3389/fonc.2021.731260. eCollection 2021. Front Oncol. 2021. PMID: 34631561 Free PMC article.
-
The Spleen Tyrosine Kinase Inhibitor, Entospletinib (GS-9973) Restores Chemosensitivity in Lung Cancer Cells by Modulating ABCG2-mediated Multidrug Resistance.Int J Biol Sci. 2021 Jun 22;17(10):2652-2665. doi: 10.7150/ijbs.61229. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326700 Free PMC article.
-
BATF2 inhibits the stem cell-like properties and chemoresistance of gastric cancer cells through PTEN/AKT/β-catenin pathway.Theranostics. 2024 Oct 21;14(18):7007-7022. doi: 10.7150/thno.98389. eCollection 2024. Theranostics. 2024. PMID: 39629124 Free PMC article.
References
-
- Barbuti A.M., Zhang G.-N., Gupta P., Narayanan S., Chen Z.-S. Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy. Volume 4. Elsevier; Amsterdam, The Netherlands: 2019. EGFR and HER2 Inhibitors as Sensitizing Agents for Cancer Chemotherapy; pp. 1–11.
-
- Gupta P., Narayanan S., Yang D.-H. Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy. Volume 4. Elsevier; Amsterdam, The Netherlands: 2019. CDK Inhibitors as Sensitizing Agents for Cancer Chemotherapy; pp. 125–149.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

